Question: Dr. Dhib s research group was using a wetted wall column to determine the overall mass transfer coefficient ( ) for absorption of ammonia NH3
Dr. Dhib s research group was using a wetted wall column to determine the overall mass transfer coefficient ( ) for absorption of ammonia NH3 in cold water at 2 0C which is 6 vol. percent in the air which normally has a molar mass of 28.96 g/mol. The water flow rate is equal to 1.4 times of gas flow rate. The outside gas concentration is 1.5 vol. percent. A wetted wall column looks like this:
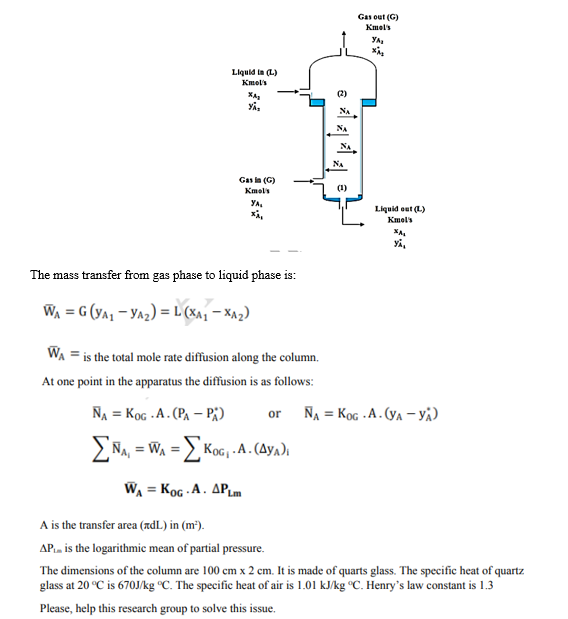
Gas out (G) Kmols YA * Liquid in (L) Kmet's XA YA, (2) NA NA NA NA (1) (1) Gas in (G) kmel's YA. xi, Liquid out (L.) Kmels YA The mass transfer from gas phase to liquid phase is: WA = G(YA, - y^2)=L(X^2 - x^2) WA = is the total mole rate diffusion along the column. At one point in the apparatus the diffusion is as follows: NA = Kog.A. (PA-P) NA = Kog.A.(YA-y) , = Wa = { Koc, .A. (Aya) or WA = KG.A. APL A is the transfer area (dL) in (m). AP.. is the logarithmic mean of partial pressure. The dimensions of the column are 100 cm x 2 cm. It is made of quarts glass. The specific heat of quartz glass at 20C is 670J/kg C. The specific heat of air is 1.01 kJ/kg "C. Henry's law constant is 1.3 Please, help this research group to solve this issue
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


