Question: Draw the complete ground - state electron configuration ( with electron spin ) of the following elements: a ) Potassium b ) Aluminum c )
Draw the complete groundstate electron configuration with electron spin of the following elements:
a Potassium
b Aluminum
c Iodine
Considered the following molecules: Draw the valence electron configuration of each atom including their spin direction and circle each pair of electrons that make a bond within the molecule. Determine the hybridization state for each carbon atom. Then draw the Lewis Structure and count how many bonds are sigma or pi bonds
a
b
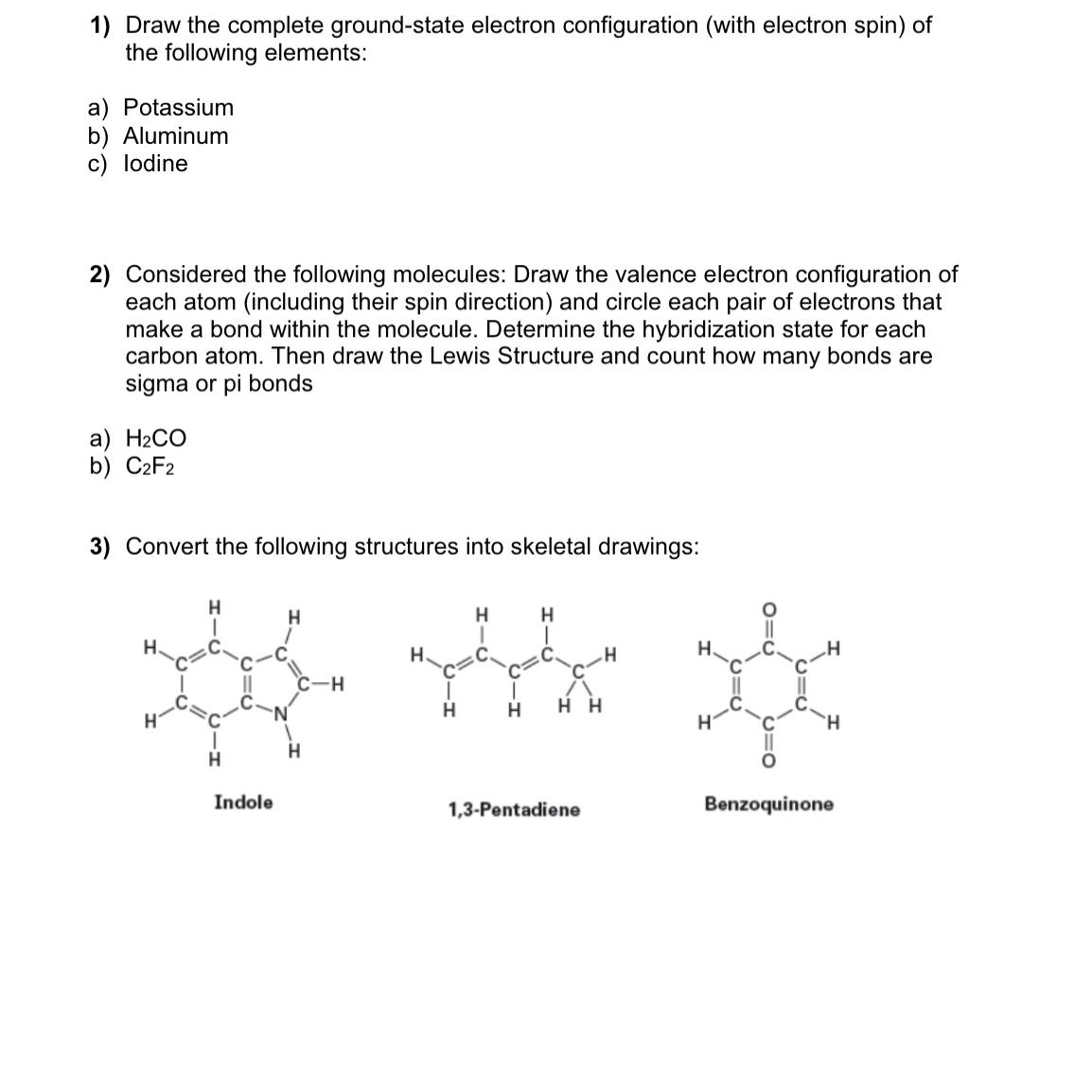
Convert the following structures into skeletal drawings:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


