Question: Draw the structure of the compound that is consistent with the H NMR data below. (Assume that long-range coupling is not observed.) *Suggestion: Find
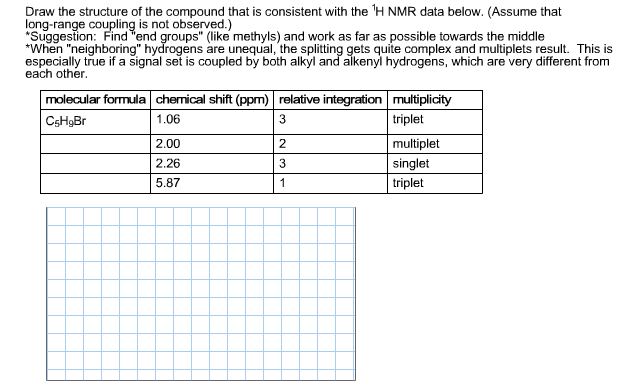
Draw the structure of the compound that is consistent with the H NMR data below. (Assume that long-range coupling is not observed.) *Suggestion: Find "end groups" (like methyls) and work as far as possible towards the middle *When "neighboring" hydrogens are unequal, the splitting gets quite complex and multiplets result. This is especially true if a signal set is coupled by both alkyl and alkenyl hydrogens, which are very different from each other. molecular formula chemical shift (ppm) relative integration multiplicity CsHgBr 1.06 3 triplet multiplet singlet triplet 2.00 2.26 5.87 2 3 1
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


