Question: Draw three-dimensional formulas for the following molecules using bold and dashed wedge bonds where appropriate. Indicate whether each bond in it is a or bond,
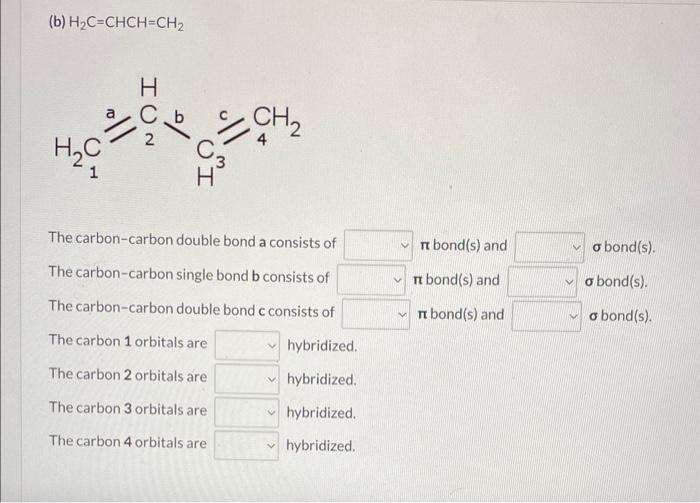
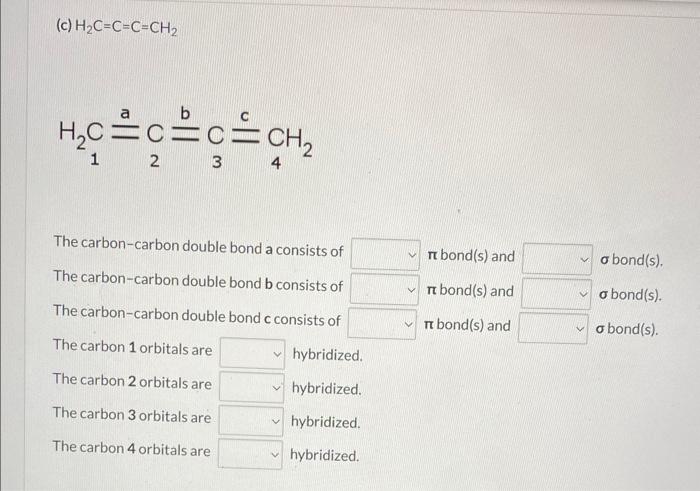
Draw three-dimensional formulas for the following molecules using bold and dashed wedge bonds where appropriate. Indicate whether each bond in it is a or bond, and provide the hybridization for each non-hydrogen atom. (a) CH2O The carbon-oxygen double bond consists of bond(s) and bond(s). The carbon orbitals are hybridized and the oxygen orbit no hybridized. two three four H2C=CHCH=CH2 (b) H2C=CHCH=CH2 Thecarbon-carbondoublebondaconsistsofThecarbon-carbonsinglebondbconsistsofThecarbon-carbondoublebondcconsistsofbond(s)andbond(s)andbond(s)andbond(s).bond(s).bond(s). The carbon 1 orbitals are hybridized. The carbon 2 orbitals are hybridized. The carbon 3 orbitals are hybridized. The carbon 4 orbitals are hybridized. H2C=C=C=CH2 (c) H2C=C=C=CH2 H2C2C2C=4C3=CH2 \begin{tabular}{l|l|l} The carbon-carbon double bond a consists of & bond(s) and & bond(s). \\ The carbon-carbon double bond b consists of & bond(s) and & bond(s). \\ The carbon-carbon double bond c consists of & bond(s) and & bond(s). \end{tabular} The carbon 1 orbitals are hybridized. The carbon 2 orbitals are hybridized. The carbon 3 orbitals are hybridized. The carbon 4 orbitals are hybridized
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


