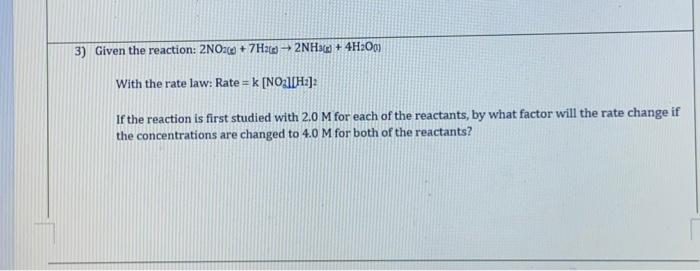
Question: e) Using the experiment 1 data, calculate the value for k, with units Given the reaction: 2NO2(+7H2()2NH3(n)+4H2O() With the rate law: Rate =k[NO3UH2]2 If the
![with units Given the reaction: 2NO2(+7H2()2NH3(n)+4H2O() With the rate law: Rate =k[NO3UH2]2](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8d7fc02500_25166f8d7fb8f3d3.jpg)
Given the reaction: 2NO2(+7H2()2NH3(n)+4H2O() With the rate law: Rate =k[NO3UH2]2 If the reaction is first studied with 2.0M for each of the reactants, by what factor will the rate change if the concentrations are changed to 4.0M for both of the reactants? 5) Given the reaction: Cr2O72(gg)+3HNO2(ag)+5H+(ag)2Cr3+(ag)+3NO3(gg)+4H2O(r) A student studies the kinetics of the reaction and their data is shown below: b) What is the order of reaction with respect to HNO2 ? c) What is the order of reaction with respect to H.? d) What is the overall rate law
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


