Question: Eceil = E + 1 in (0,1)+(a)). (aH) 2F Such a fuel cell operates at a system pressure of 1 MPa and temperature of
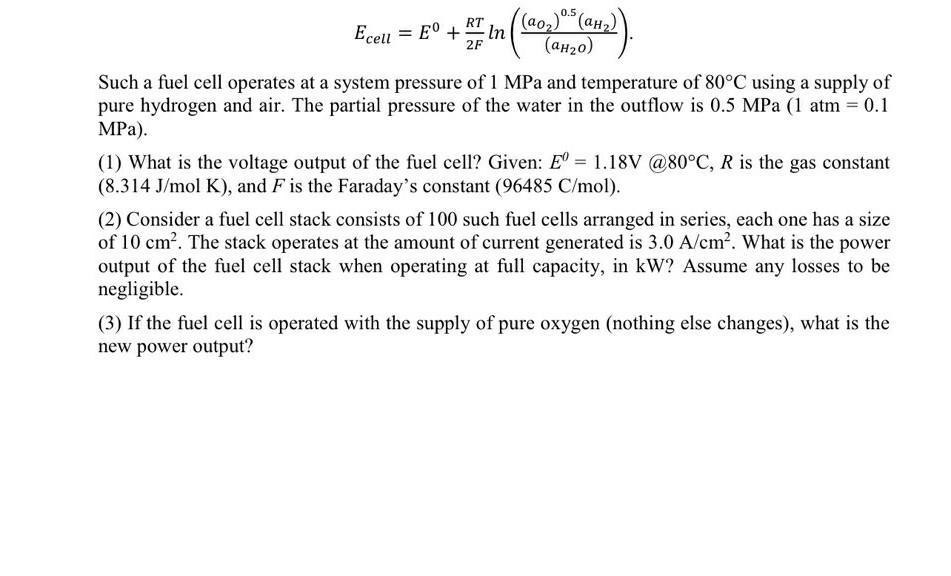
Eceil = E + 1 in (0,1)+(a)). (aH) 2F Such a fuel cell operates at a system pressure of 1 MPa and temperature of 80C using a supply of pure hydrogen and air. The partial pressure of the water in the outflow is 0.5 MPa (1 atm = 0.1 MPa). (1) What is the voltage output of the fuel cell? Given: E= 1.18V @80C, R is the gas constant (8.314 J/mol K), and F is the Faraday's constant (96485 C/mol). (2) Consider a fuel cell stack consists of 100 such fuel cells arranged in series, each one has a size of 10 cm. The stack operates at the amount of current generated is 3.0 A/cm. What is the power output of the fuel cell stack when operating at full capacity, in kW? Assume any losses to be negligible. (3) If the fuel cell is operated with the supply of pure oxygen (nothing else changes), what is the new power output?
Step by Step Solution
3.48 Rating (151 Votes )
There are 3 Steps involved in it
Given H 021220 P10 05 MPa 5atm Total Systems pressure Temperature JoC R 8... View full answer

Get step-by-step solutions from verified subject matter experts


