Question: Based on the single-step global mechanism given by Eqns. 5.1 and 5.2 (page 156), write down the chemical reaction for octane combustion at equivalence

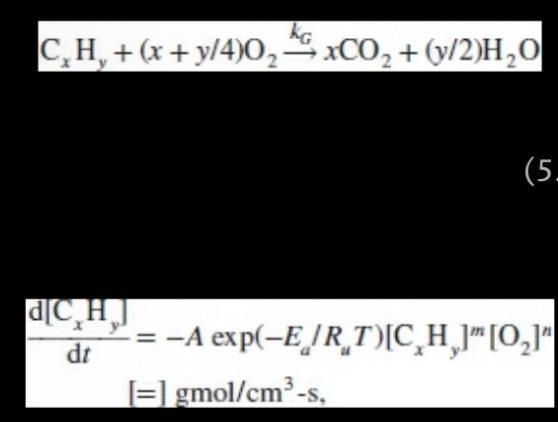
Based on the single-step global mechanism given by Eqns. 5.1 and 5.2 (page 156), write down the chemical reaction for octane combustion at equivalence ratio of 1.0 and then determine the molar concentrations of octane and oxygen in units of gmol/cm. CH + (x + y/4)0xCO+ (v/2)HO d[CH] y dt (5. -=-A exp(-E/RT)[CH] [0]" [=] gmol/cm-s,
Step by Step Solution
3.49 Rating (166 Votes )
There are 3 Steps involved in it
To obtain the molar concentration of octane and oxygen in gmolcm 3 for the combustion of octane C 8 ... View full answer

Get step-by-step solutions from verified subject matter experts


