Question: Electrodes Half-reactions E E cell Zn Cu - Zn(s) - Zn2+ + 2e Cu2+ + 2e + Cu(s) Tou(s) Cu+ + 2 2 +1.10 V
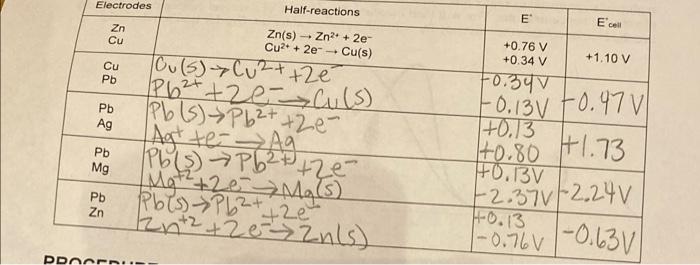
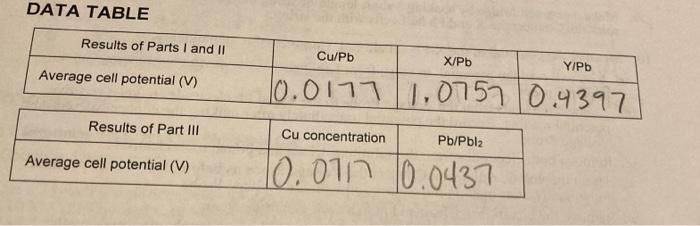
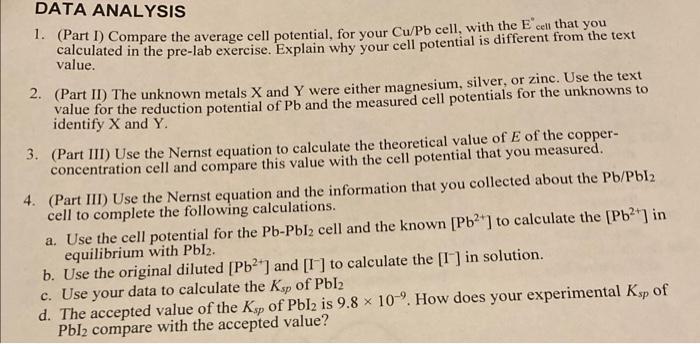
Electrodes Half-reactions E E cell Zn Cu - Zn(s) - Zn2+ + 2e Cu2+ + 2e + Cu(s) Tou(s) Cu+ + 2 2 +1.10 V Cu Pb +0.76 V +0.34 V 50.347 Pb Ag +0.13 Pb Mg Pb+ +zculs Pb (s) Pb2+ +2e- Agt te Aa Pb(s) Pb2+ +ze Mg2+261 Mg(s) Pbls) Pb2+ ze 2n+2 +2e Zuls) -0.13v 70.47 +0.80 +1.73 -2.37v / 2,24v 1-0.76 -0.630 HO.13V Pb Zn +2e 40.13 | PDOEN.. DATA TABLE Results of Parts I and II Cu/Pb X/Pb Y/Pb Average cell potential (V) 10.01771.0757 0.4397 Results of Part III Cu concentration Pb/Pbla Average cell potential (V) To.070 10.0437 DATA ANALYSIS 1. (Part 1) Compare the average cell potential, for your Cu/Pb cell, with the cell that you calculated in the pre-lab exercise. Explain why your cell potential is different from the text 2. (Part II) The unknown metals X and Y were either magnesium, silver, or zinc. Use the text value for the reduction potential of Pb and the measured cell potentials for the unknowns to identify X and Y. 3. (Part III) Use the Nernst equation to calculate the theoretical value of E of the copper- concentration cell and compare this value with the cell potential that you measured. 4. (Part III) Use the Nernst equation and the information that you collected about the Pb/Pb12 cell to complete the following calculations. a. Use the cell potential for the Pb-Pbl2 cell and the known (Pb) to calculate the [Pb2') in equilibrium with Pbl. b. Use the original diluted [Pb2+] and [I ] to calculate the [I ) in solution. c. Use your data to calculate the Ksp of Pbl2 d. The accepted value of the Ksp of Pblu is 9.8 x 10-'. How does your experimental Ksp of Pbl2 compare with the accepted value
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


