Question: elementary steps consistent with a rate law. Step 1. Define. A reaction does not just happen all at once. It is similar to an assembly
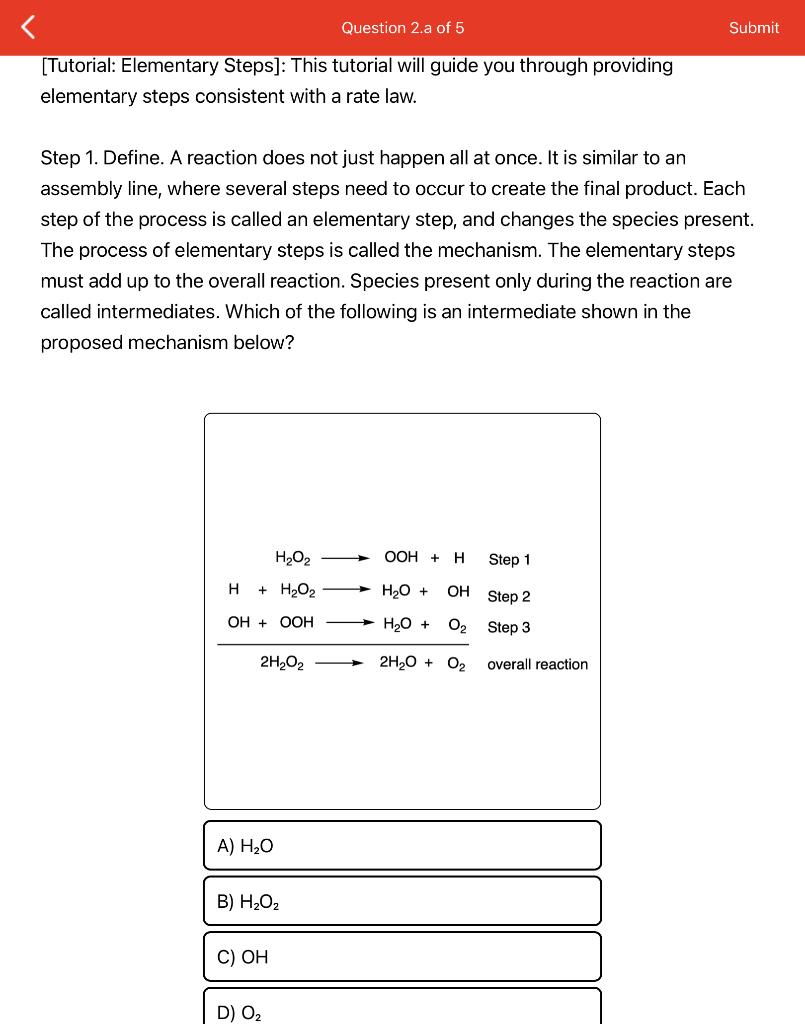
elementary steps consistent with a rate law. Step 1. Define. A reaction does not just happen all at once. It is similar to an assembly line, where several steps need to occur to create the final product. Each step of the process is called an elementary step, and changes the species present. The process of elementary steps is called the mechanism. The elementary steps must add up to the overall reaction. Species present only during the reaction are called intermediates. Which of the following is an intermediate shown in the proposed mechanism below
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


