Question: Energy and Enthalpy Changes, Heat and Work -- Monatomic Ideal Gas 2.00-mol of a monatomic ideal gas goes from State A to State D via
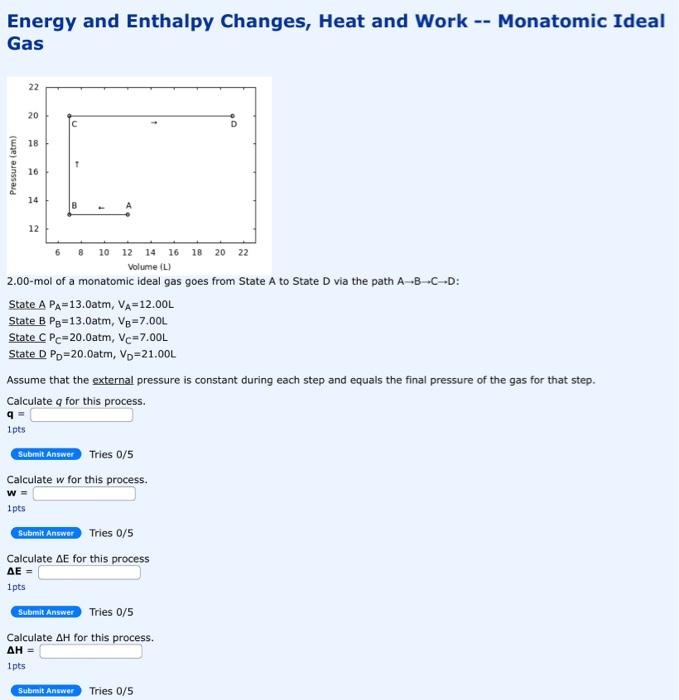
Energy and Enthalpy Changes, Heat and Work -- Monatomic Ideal Gas 2.00-mol of a monatomic ideal gas goes from State A to State D via the path ABCD : State APA=13.0atm,VA=12.00L State BPB=13.0atm,VB=7.00L State CPC=20.0atm,VC=7.00L State DPD=20.0atm,VD=21.00L Assume that the external pressure is constant during each step and equals the final pressure of the gas for that step. Calculate q for this process. q= 1pts Tries 0/5 Calculate w for this process. w= 1pts Tries 0/5 Calculate E for this process E= 1pts Tries 0/5 Calculate H for this process. H= 1pts Tries 0/5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


