Question: Energy and Enthalpy Changes, Heat and Work -- Monatomic Ideal Gas 2.00-mol of a monatomic ideal gas goes from State A to State D via
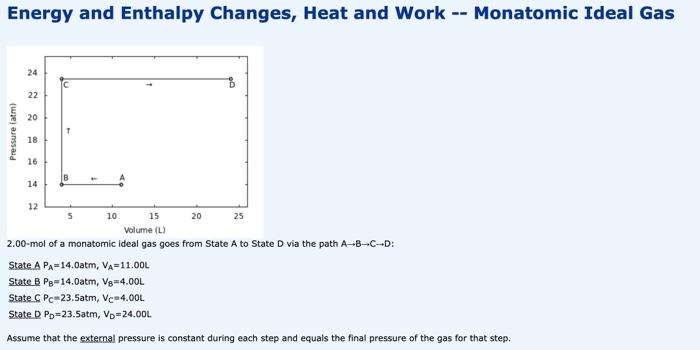
Energy and Enthalpy Changes, Heat and Work -- Monatomic Ideal Gas 2.00-mol of a monatomic ideal gas goes from State A to State D via the path ABCD : State APA=14.0atm,VA=11.00L State BPB=14.0atm,VB=4.00L State CPC=23.5atm,VC=4.00L State. DPD=23.5atm,VD=24.00L Assume that the external pressure is constant during each step and equals the final pressure of the gas for that step. Calculate q for this process. q= 1pts Tries 0/10 Calculate w for this process. w= 1pts Tries 0/10 Calculate E for this process E= 1 pts Tries 0/10 Calculate H for this process. H= 1pts Tries 0/10
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


