Question: Enter your answer in the provided box. A nonvolatile organic compound Z was used to make up a solution. Solution A contains 10.00g of Z


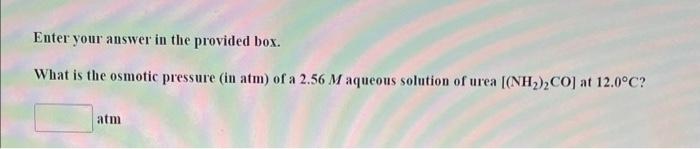
Enter your answer in the provided box. A nonvolatile organic compound Z was used to make up a solution. Solution A contains 10.00g of Z dissolved in 100g of water and has a vapor pressure of 754.5mmHg at the normal boiling point of water: Calculate the molar mass of Z in solution A. What are the boiling point and freezing point of a 5.47m solution of naphthalene in benzene? (The boiling point and freezing point of benzene are 80.1C and 5.5C respectively. The boiling point elevation constant for benzene is 2.53C/m, and the freezing point depression constant for benzene is 5.12C/m.) Boiling point C Freezing point = C Enter your answer in the provided box. What is the osmotic pressure (in atm) of a 2.56M aqueous solution of urea [(NH2)2CO] at 12.0C ? atm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


