Question: Enter your answer in the provided box. Calculate the molality of a 44.3 percent (by mass) aqueous solution of sodium chloride. m Enter your answer
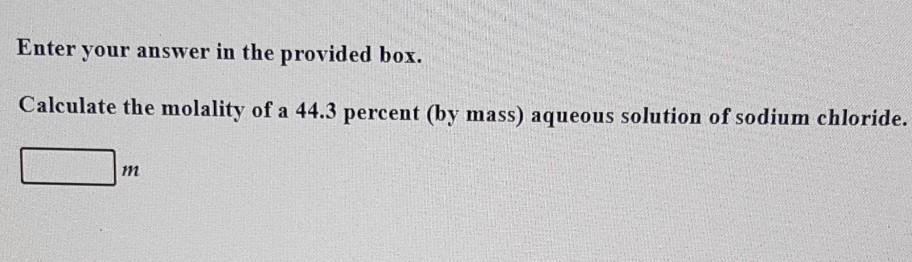


Enter your answer in the provided box. Calculate the molality of a 44.3 percent (by mass) aqueous solution of sodium chloride. m Enter your answer in the provided box. A sample of 0.7560 g of an unknown compound containing barium ions (Ba?') is dissolved in water and treated with an excess of Na2SO4. If the mass of the BaSO4 precipitate formed is 0.9445 g, what is the percent by mass of Ba in the original unknown compound? % Be sure to answer all parts. How many H atoms are in 75.7 g of isopropanol (rubbing alcohol), C3H2O? Enter your answer in scientific notation. X 10! H atoms
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


