Question: Esters (i) aliphatic: RCOR' M+ weak but observable note: M+ gets less intense as the alcohol portion ( ) increases in size: e.g. C4
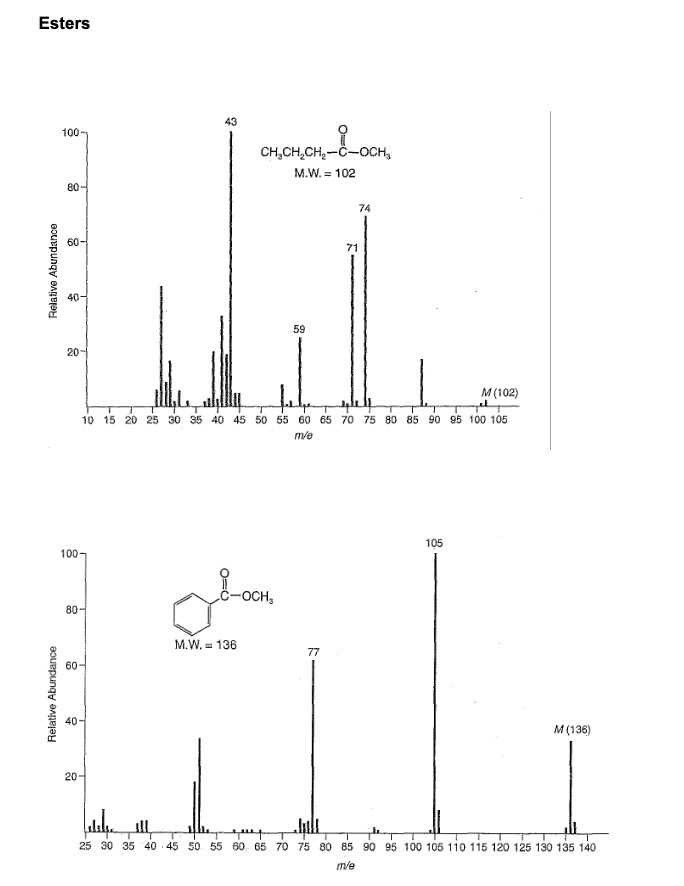
Esters (i) aliphatic: RCOR' M+ weak but observable note: M+ gets less intense as the alcohol portion ( ) increases in size: e.g. C4 very weak. M-R M - OR' 0+ R-C-O-R + R-C-O-R' Which peaks in the MS of methyl butanoate and methyl benzoate are due to these types of fragmentation ? Account for the peak at m/z = 74 in the methyl butanoate MS. Esters Relative Abundance Relative Abundance 100 80 60- 40- 20 100- 80- 60 40 43 20- CHCHCH-C-OCH M.W. = 102 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 105 m/e 3-com. M.W. 136 59 71 77 74 M (102) 105 M (136) elekea ill T quep T 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 105 110 115 120 125 130 135 140 m/e
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it
Fragmentation pat... View full answer

Get step-by-step solutions from verified subject matter experts


