Question: Ethylene oxide ( C 2 H 4 O ) is a high volume chemical intermediate that is used to produce glycol and polyethylene glycol. Ethylene
Ethylene oxide is a high volume chemical intermediate that is used to produce
glycol and polyethylene glycol. Ethylene oxide is produced by the partial oxidation of
ethylene using a solid catalyst in a fixed bed reactor:
Unfortunately, a portion of the ethylene reacts completely to and :
The product gas leaving a fixed bed ethylene oxide reactor has the following water free
composition: and Determine the percent
excess air used in the reactor, based on the desired reaction and the mass of ethylene feed
in required to produce tonsyear of ethylene oxide.
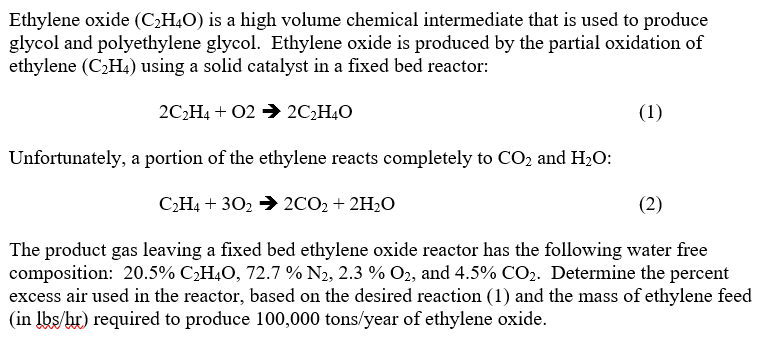
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


