Question: Every electron in an atom is described by a unique set of four quantum numbers: n,,m, and ms. The principal quantum number, n, identifies the
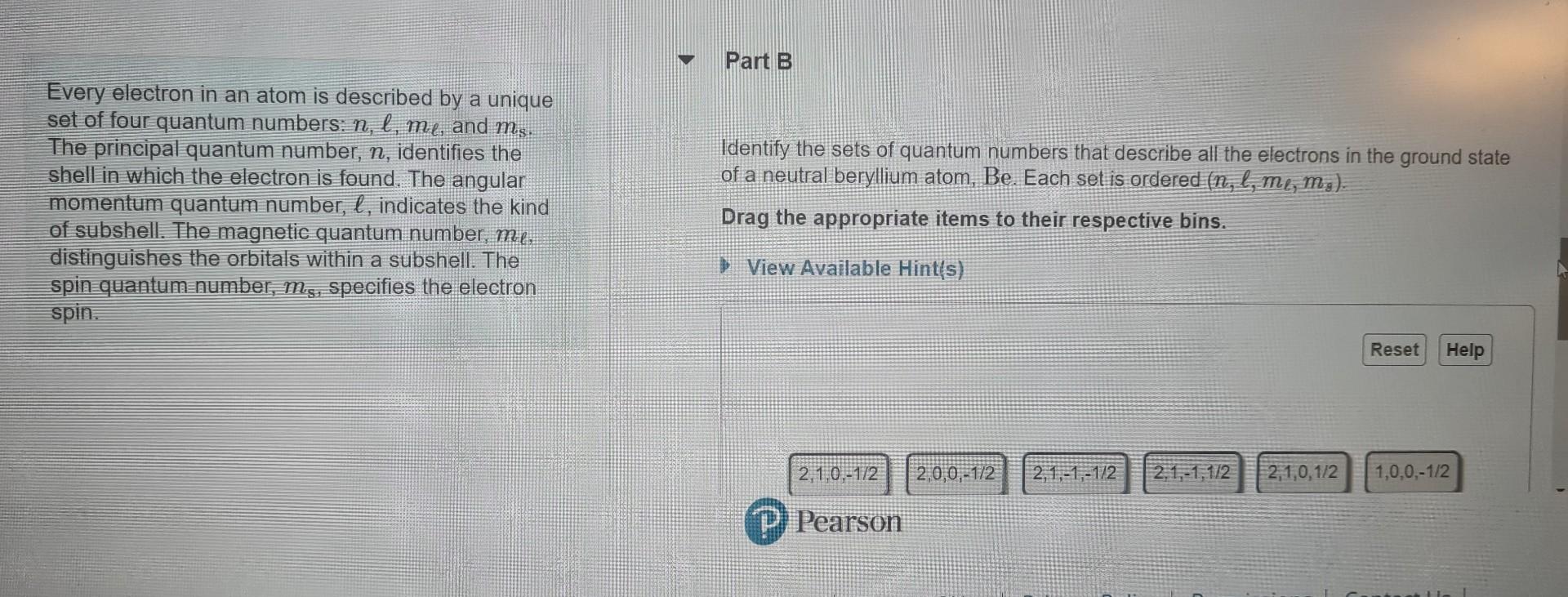
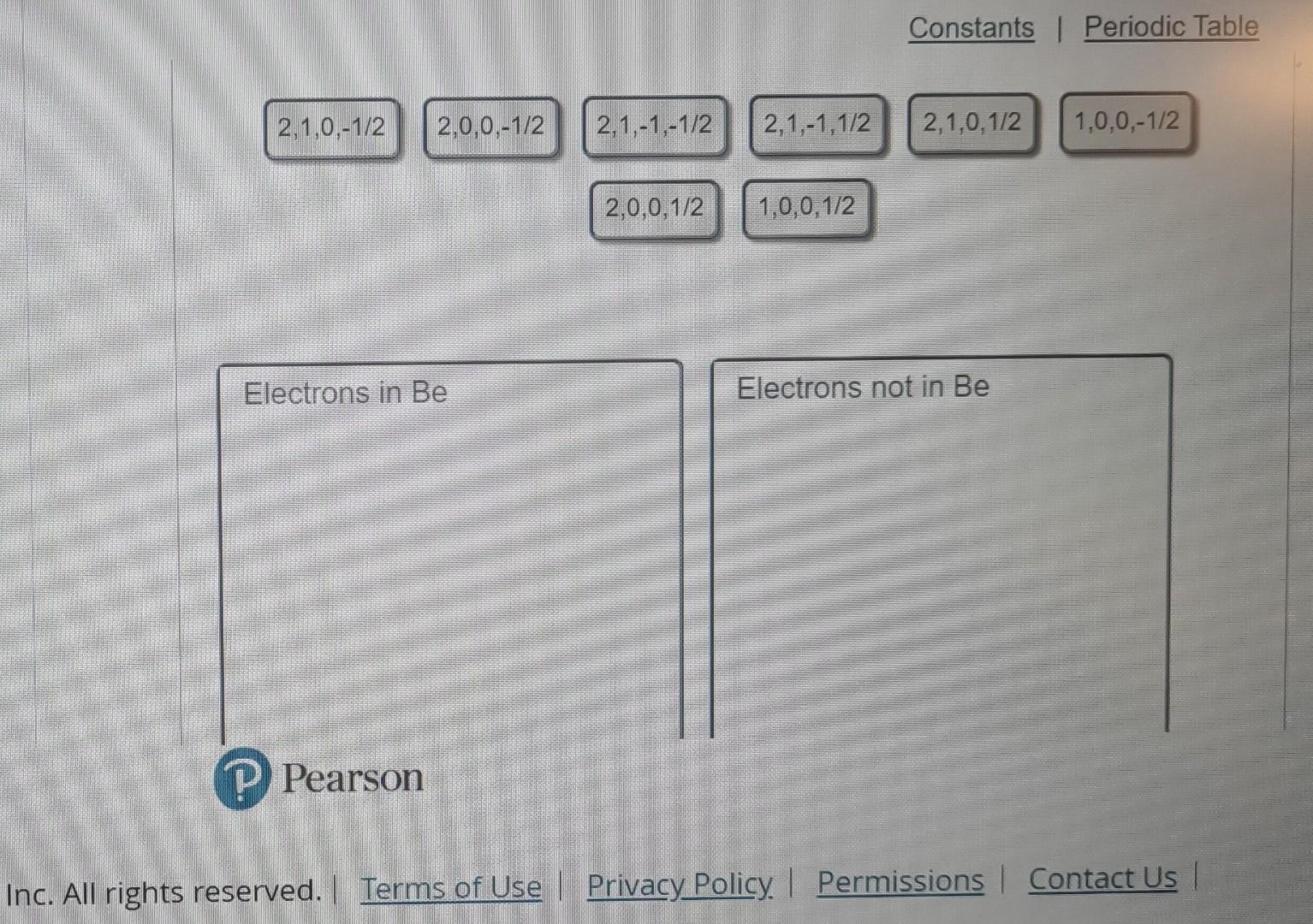
Every electron in an atom is described by a unique set of four quantum numbers: n,,m, and ms. The principal quantum number, n, identifies the Identify the sets of quantum numbers that describe all the electrons in the ground state shell in which the electron is found. The angular of a neutral beryllium atom, Be. Each set is ordered (n,,,m,m8). momentum quantum number, , indicates the kind of subshell. The magnetic quantum number, m. distingulishes the orbitals within a subshell. The Drag the appropriate items to their respective bins. spin quantumnumber, ms, specifies the electron spin. Inc. All righirs ieserveu
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


