Question: Example 3 . CSTR Example A + B P Two streams are feeding the reactor. One concentrated feed with flow rate F 1 ( m
Example CSTR Example
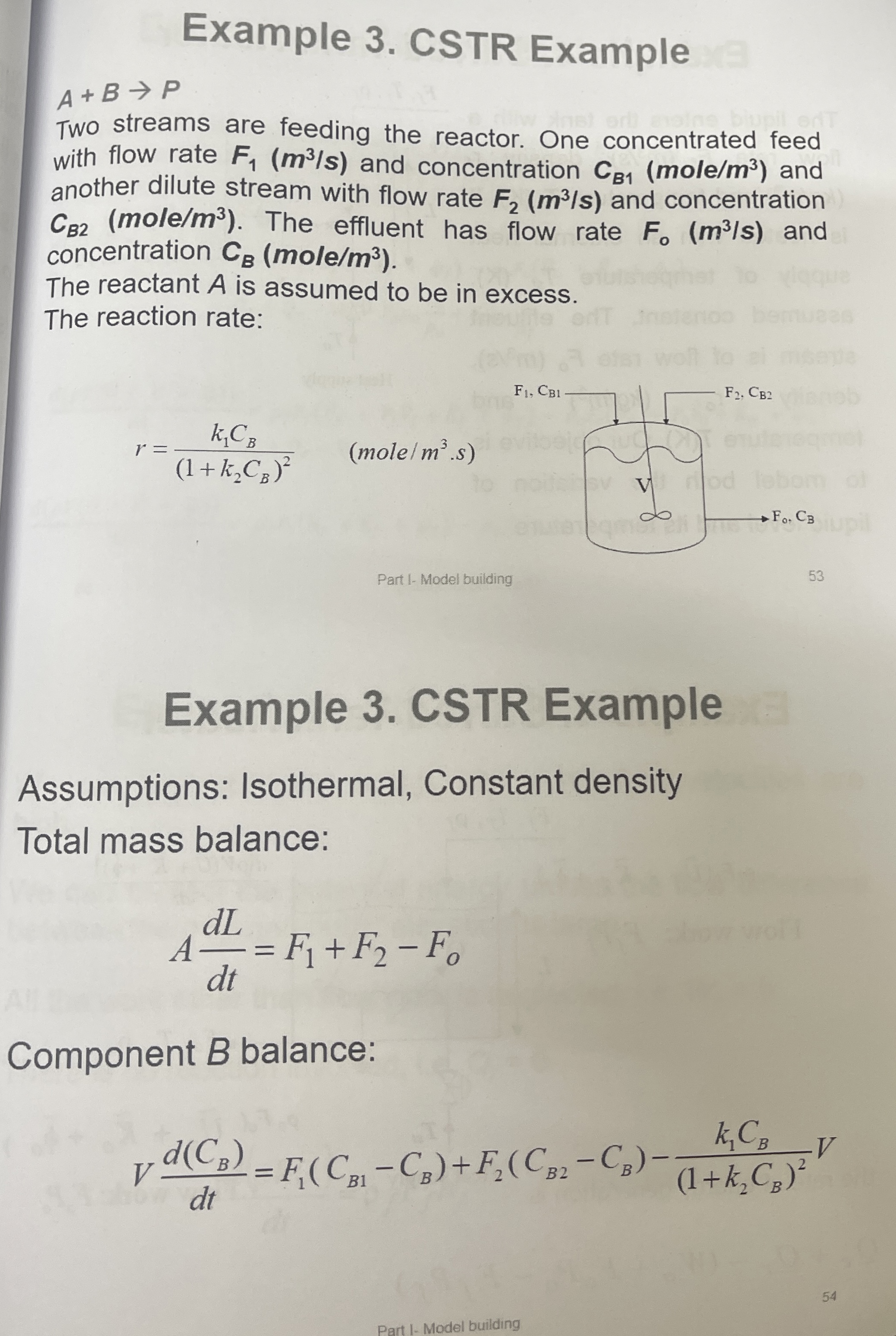
Two streams are feeding the reactor. One concentrated feed with flow rate and concentration and another dilute stream with flow rate and concentration The effluent has flow rate and concentration mole
The reactant is assumed to be in excess.
The reaction rate:
mole
Part Model building
Example CSTR Example
Assumptions: Isothermal, Constant density Total mass balance:
Component balance:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


