Question: Example 3 ln(dtdCA)=ln(k)+ln(CA) Reaction: AP Table 1: Experimental data (b) Determine the rate constant (k) and the reaction order with respect to A using differential
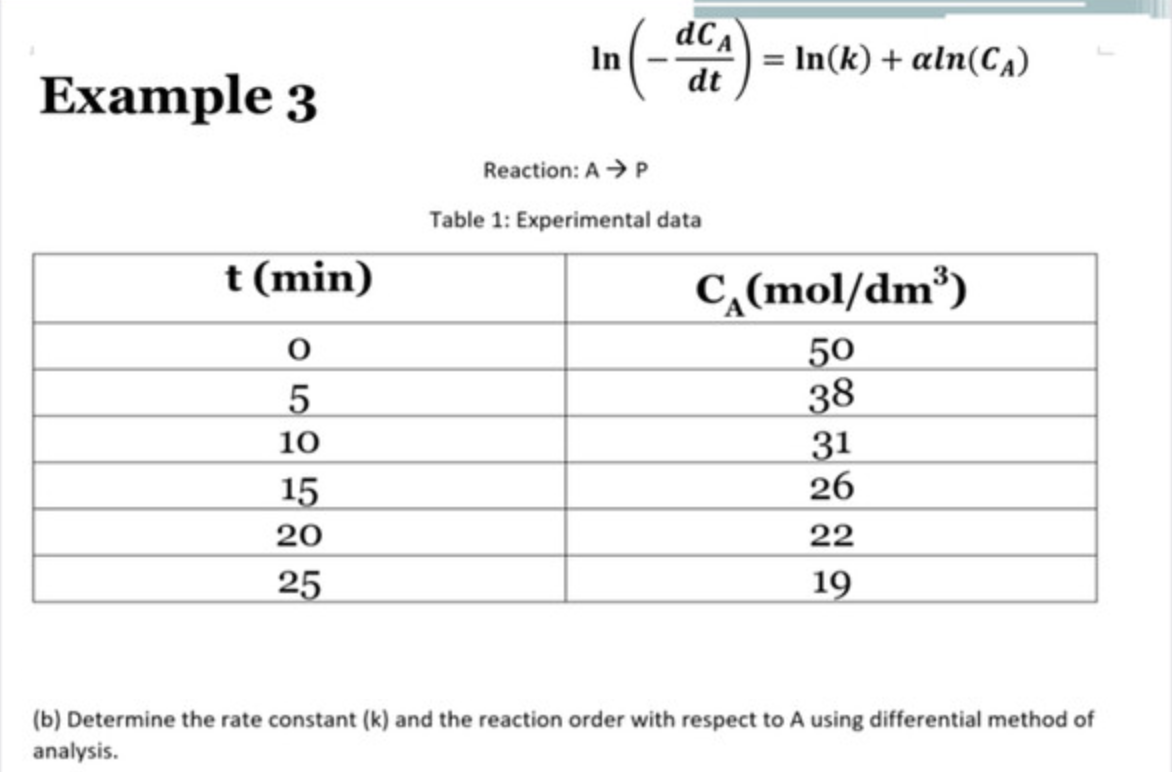
Example 3 ln(dtdCA)=ln(k)+ln(CA) Reaction: AP Table 1: Experimental data (b) Determine the rate constant (k) and the reaction order with respect to A using differential method of analysis
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


