Question: help me with only with question b. A- A x Aty Question 4 Reactant A enters the reactor at volumetric flow rate of 10 dm?/min.
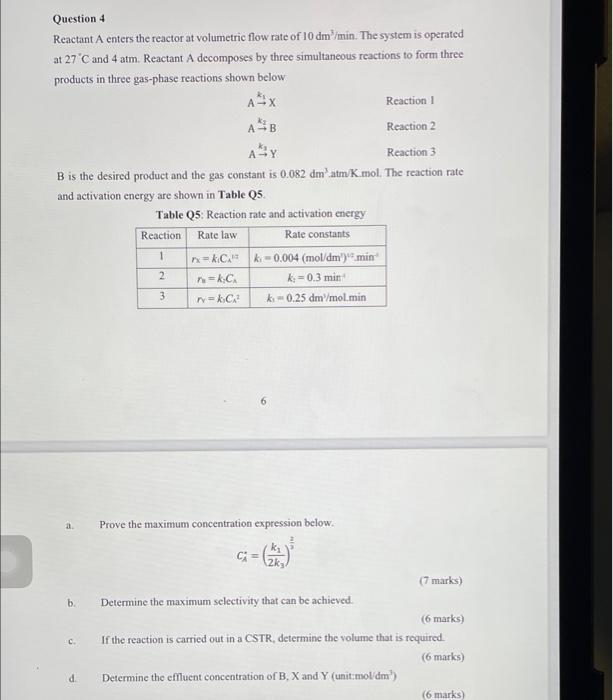
A- A x Aty Question 4 Reactant A enters the reactor at volumetric flow rate of 10 dm?/min. The system is operated at 27C and 4 atm. Reactant A decomposes by three simultaneous reactions to form three products in three gas-phase reactions shown below Reaction Reaction 2 Reaction 3 B is the desired product and the gas constant is 0.082 dm.atm/K.mol. The reaction rate and activation energy are shown in Table Q5 Table Q5: Reaction rate and activation energy Reaction Rate law Rate constants n-C k-0.004 (mol/dm) min =k.c k;= 0.3 min rakc -0.25 dm /mol.min 1 2 3 2 Prove the maximum concentration expression below. G= b. 2k (7 marks) Determine the maximum selectivity that can be achieved. (6 marks) If the reaction is carried out in a CSTR, determine the volume that is required. (6 marks) Determine the effluent concentration of B, X and Y (unit:moldm) (6 marks) C d
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


