Question: Example SA. 1 Determining a partial molar volume A polynomial fit to measurements of the total volume of a water) ethanol mixture at 25C that
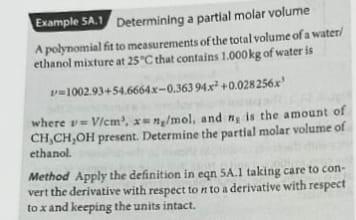
Example SA. 1 Determining a partial molar volume A polynomial fit to measurements of the total volume of a water) ethanol mixture at 25C that contains 1.000 kg of water is v=1002.93+54.6664x-0.36394x* +0.028256x! where v=V/cm', **/mol, and ng is the amount of CH,CH,OH present. Determine the partial molar volume of ethanol Method Apply the definition in en 5A.1 taking care to con- vert the derivative with respect to n to a derivative with respect to x and keeping the units intact
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


