Question: Exercise 10.1 Use the VSEPR method to predict the ge- ometry of the following ion and molecules: a. CIO,; b. OF; c. SiF4 1. ion
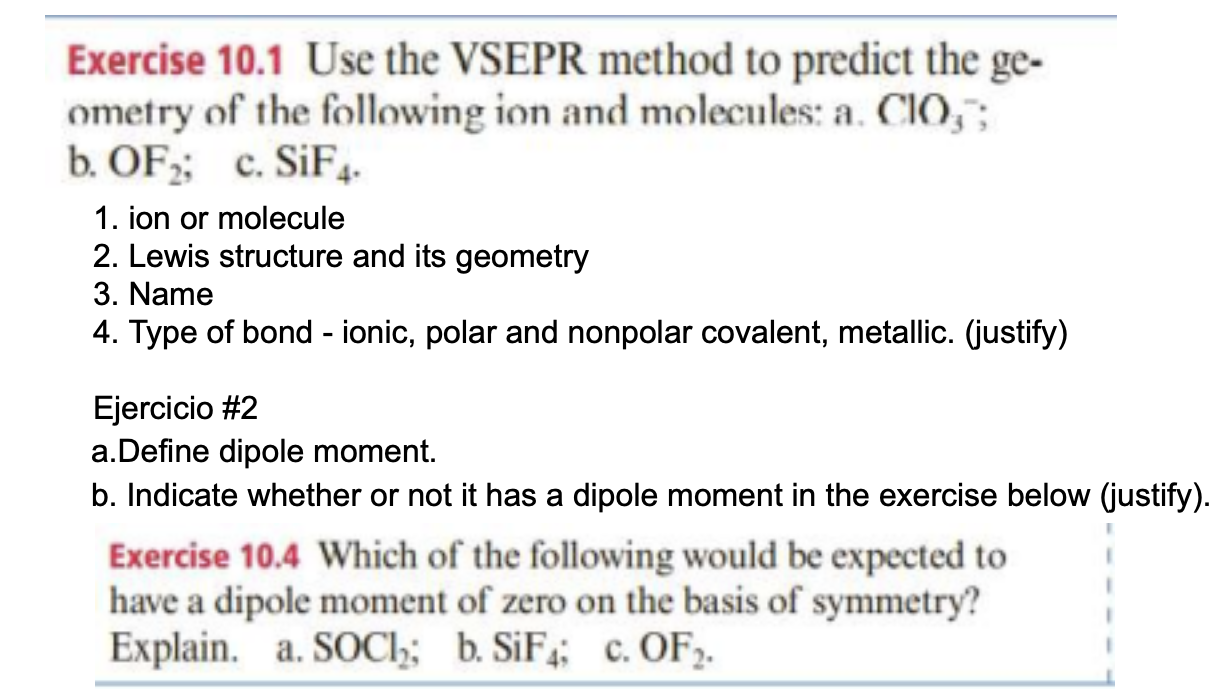
Exercise 10.1 Use the VSEPR method to predict the ge- ometry of the following ion and molecules: a. CIO,; b. OF; c. SiF4 1. ion or molecule 2. Lewis structure and its geometry 3. Name 4. Type of bond - ionic, polar and nonpolar covalent, metallic. (justify) Ejercicio #2 a.Define dipole moment. b. Indicate whether or not it has a dipole moment in the exercise below (justify). Exercise 10.4 Which of the following would be expected to have a dipole moment of zero on the basis of symmetry? Explain. a. SOCI; b. SiF; C. OF
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


