Question: (Exercise 2) Draw the extraction curve (log DM vs pH) on your excel file for the extraction of M(II)ion from the diluted aqueous phase into
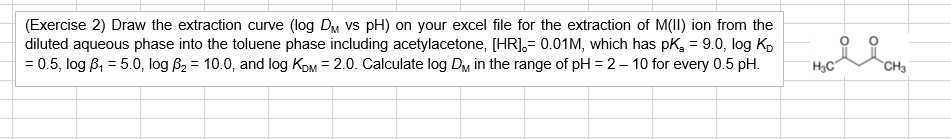
(Exercise 2) Draw the extraction curve (log DM vs pH) on your excel file for the extraction of M(II)ion from the diluted aqueous phase into the toluene phase including acetylacetone, [HR].= 0.01M, which has pk, = 9.0, log ko = 0.5, log B, = 5.0, log B2 = 10.0, and log Kom= 2.0. Calculate log Dm in the range of pH = 2 -10 for every 0.5 pH. rollin HC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


