Question: Exercise 4.4: Catalyst deactivation in a batch reactor Consider the irreversible, liquid-phase isomerization reaction carried out in a solvent containing dissolved catalyst at 25C in
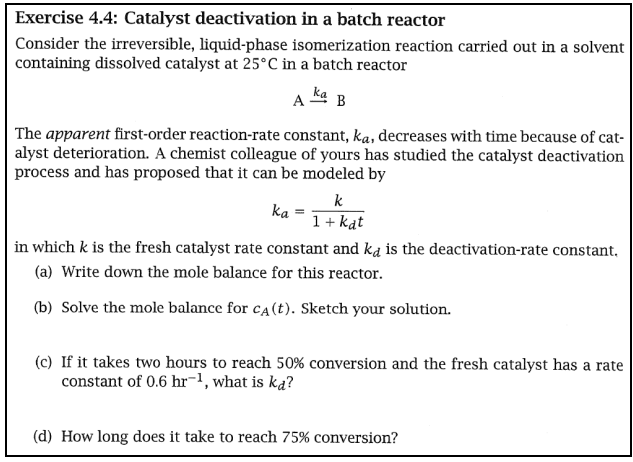
Exercise 4.4: Catalyst deactivation in a batch reactor Consider the irreversible, liquid-phase isomerization reaction carried out in a solvent containing dissolved catalyst at 25C in a batch reactor AkaB The apparent first-order reaction-rate constant, ka, decreases with time because of catalyst deterioration. A chemist colleague of yours has studied the catalyst deactivation process and has proposed that it can be modeled by ka=1+kdtk in which k is the fresh catalyst rate constant and kd is the deactivation-rate constant. (a) Write down the mole balance for this reactor. (b) Solve the mole balance for cA(t). Sketch your solution. (c) If it takes two hours to reach 50% conversion and the fresh catalyst has a rate constant of 0.6hr1, what is kd ? (d) How long does it take to reach 75% conversion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


