Question: Explain the difference between an ideal and a nonideal solution. Match the items in the left column to the appropriate blanks in the sentences on
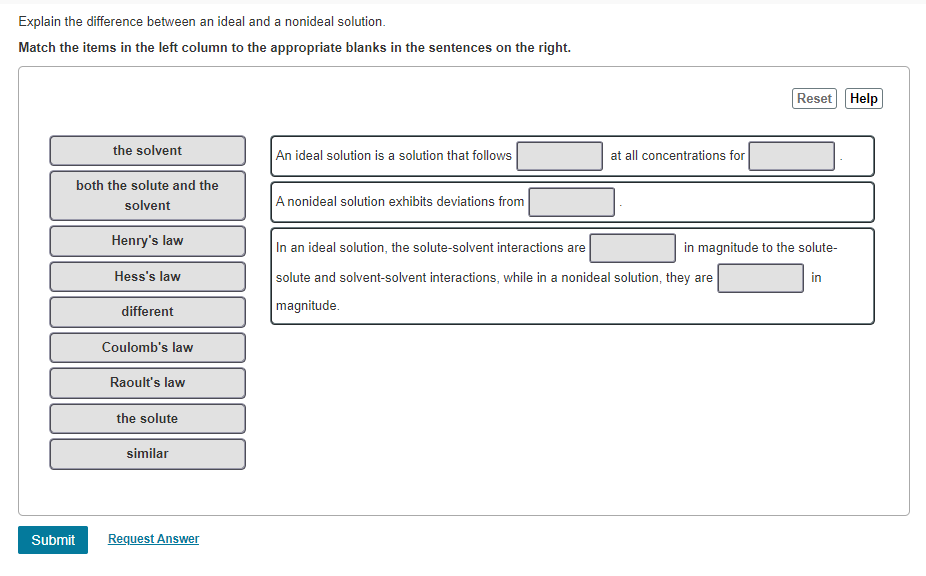
Explain the difference between an ideal and a nonideal solution. Match the items in the left column to the appropriate blanks in the sentences on the right. Reset Help the solvent An ideal solution is a solution that follows at all concentrations for both the solute and the solvent A nonideal solution exhibits deviations from Henry's law In an ideal solution, the solute-solvent interactions are in magnitude to the solute- Hess's law in solute and solvent-solvent interactions, while in a nonideal solution, they are magnitude different Coulomb's law Raoult's law the solute similar Submit Request
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


