Question: 17. For the reaction: A + B - C AH= +30 kJ/mol and AS = +50 J/K.mol. At what temperature will this reaction be
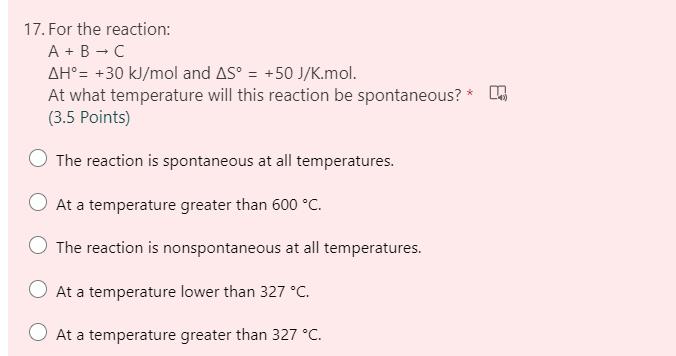
17. For the reaction: A + B - C AH= +30 kJ/mol and AS = +50 J/K.mol. At what temperature will this reaction be spontaneous? * (3.5 Points) The reaction is spontaneous at all temperatures. At a temperature greater than 600 C. The reaction is nonspontaneous at all temperatures. O At a temperature lower than 327 C. O At a temperature greater than 327 C.
Step by Step Solution
★★★★★
3.48 Rating (148 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


