Question: FeS can be roasted in 02 to form FeO: 2FeS +302 2FeO +2502 If the slag (solid product) contains 80% FeO and 20% FeS,
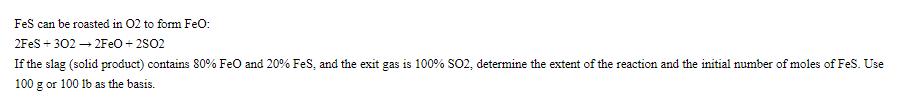 Â
Â
FeS can be roasted in 02 to form FeO: 2FeS +302 2FeO +2502 If the slag (solid product) contains 80% FeO and 20% FeS, and the exit gas is 100% SO2, determine the extent of the reaction and the initial number of moles of FeS. Use 100 g or 100 lb as the basis.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Calculate the molar masses of FeS IronII Sulfide FeO IronII Oxide and SO2 Sulfur Dioxide FeS Fe 5585 ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


