Question: Fig. 7.12. The binary system Na2SiO3SiO2. The dashed line shows metastable liquid-liquid phase separation. 2. Given 70g of SiO2 and 30g of Na2O that is
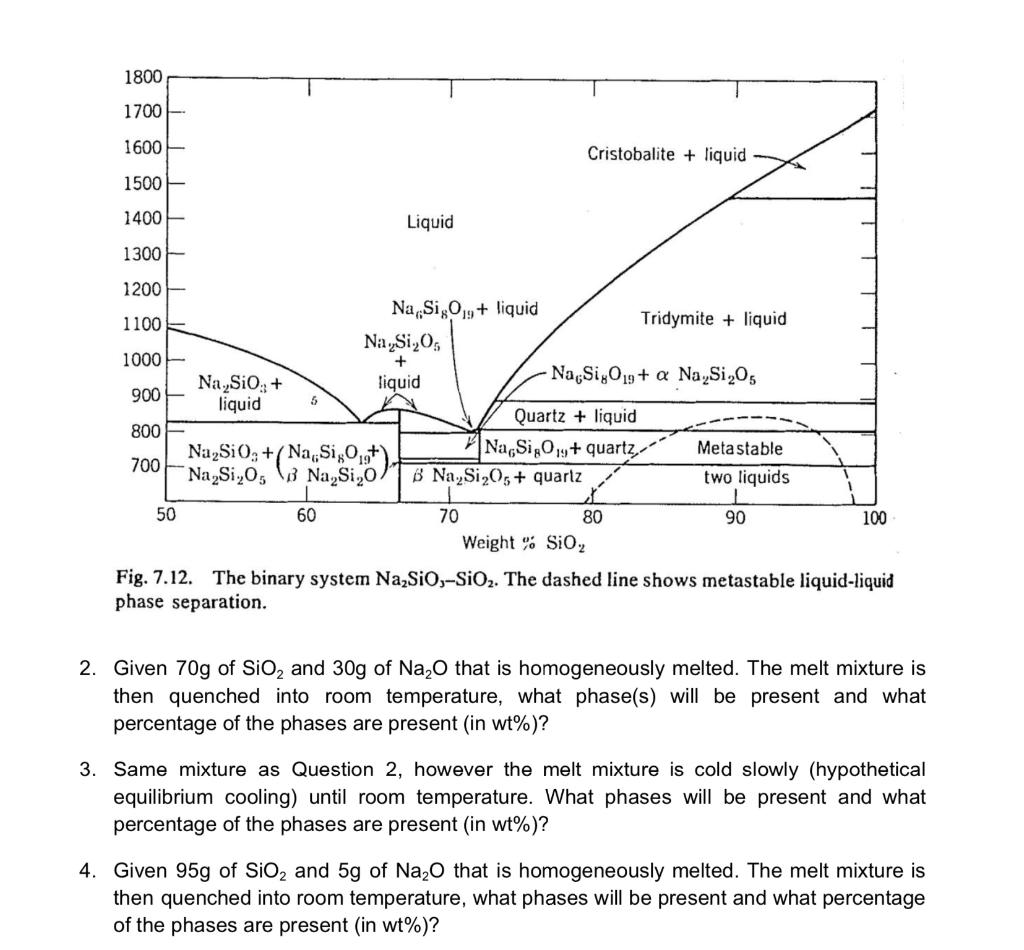
Fig. 7.12. The binary system Na2SiO3SiO2. The dashed line shows metastable liquid-liquid phase separation. 2. Given 70g of SiO2 and 30g of Na2O that is homogeneously melted. The melt mixture is then quenched into room temperature, what phase(s) will be present and what percentage of the phases are present (in wt\%)? 3. Same mixture as Question 2, however the melt mixture is cold slowly (hypothetical equilibrium cooling) until room temperature. What phases will be present and what percentage of the phases are present (in wt\%)? 4. Given 95g of SiO2 and 5g of Na2O that is homogeneously melted. The melt mixture is then quenched into room temperature, what phases will be present and what percentage of the phases are present (in wt\%)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


