Question: 6.9$ The temperature-composition diagram for the Ca/Si binary system is shown in Fig. 6.46. (a) Identify eutectics, congruent melting compounds, and incongruent melting compounds. (b)
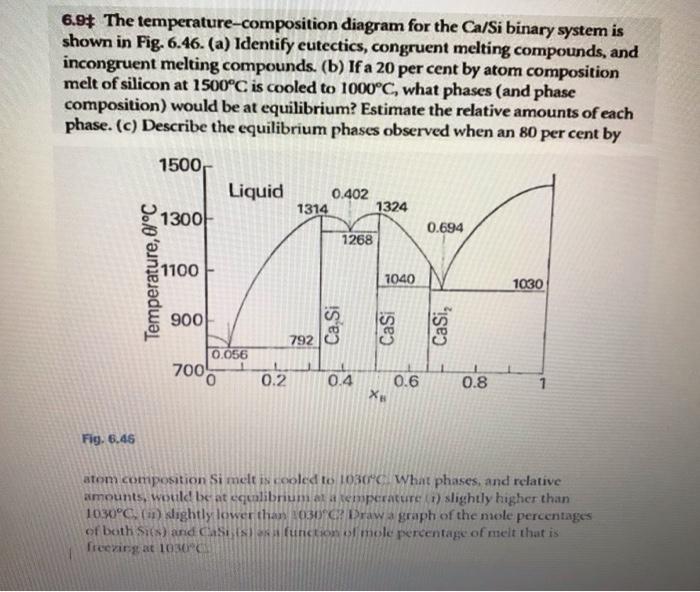
6.9\$ The temperature-composition diagram for the Ca/Si binary system is shown in Fig. 6.46. (a) Identify eutectics, congruent melting compounds, and incongruent melting compounds. (b) If a 20 per cent by atom composition melt of silicon at 1500C is cooled to 1000C, what phases (and phase composition) would be at equilibrium? Estimate the relative amounts of each phase. (c) Describe the equilibrium phases observed when an 80 per cent by Fig. 6.45 atom composition Si melt is rooled 101030C. What phases, and relative amounts, wotk be at equlibrium of a semperature (t) slightly higher than 1030C, (it) Wightly lower than 03)G. Draw a graph of the noole percentages of both sies) and casits) frin function of mole percentage of meit that is frecrimgat 1030 6.9\$ The temperature-composition diagram for the Ca/Si binary system is shown in Fig. 6.46. (a) Identify eutectics, congruent melting compounds, and incongruent melting compounds. (b) If a 20 per cent by atom composition melt of silicon at 1500C is cooled to 1000C, what phases (and phase composition) would be at equilibrium? Estimate the relative amounts of each phase. (c) Describe the equilibrium phases observed when an 80 per cent by Fig. 6.45 atom composition Si melt is rooled 101030C. What phases, and relative amounts, wotk be at equlibrium of a semperature (t) slightly higher than 1030C, (it) Wightly lower than 03)G. Draw a graph of the noole percentages of both sies) and casits) frin function of mole percentage of meit that is frecrimgat 1030
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


