Question: Balance the following equation for the combustion of benzene: CH() + O2(g) HO(g) + CO(g) a) CH() + 902(g) + 3H2O(g) + 6CO2(g) b)
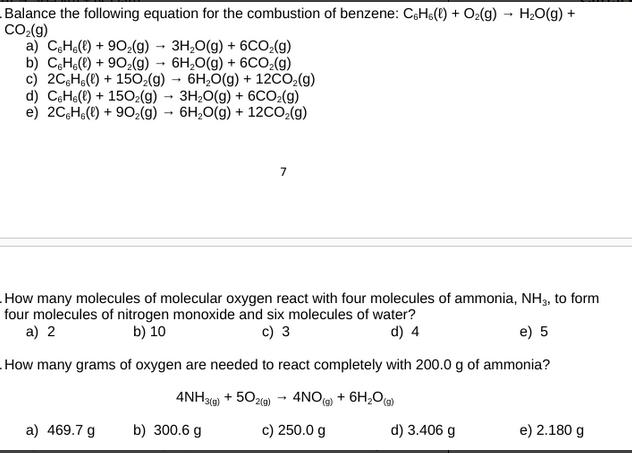
Balance the following equation for the combustion of benzene: CH() + O2(g) HO(g) + CO(g) a) CH() + 902(g) + 3H2O(g) + 6CO2(g) b) CH()+902(g) 6HO(g) + 6CO2(g) c) 2CH() + 1502(g) 6H2O(g) + 12CO2(g) d) CH(l) +1502(g) 3H2O(g) + 6CO2(g) e) 2CH() + 902(g) 6H2O(g) + 12CO2(g) 7 How many molecules of molecular oxygen react with four molecules of ammonia, NH3, to form four molecules of nitrogen monoxide and six molecules of water? a) 2 b) 10 c) 3 d) 4 e) 5 How many grams of oxygen are needed to react completely with 200.0 g of ammonia? 4NH3(g) + 502(9) 4NO(g) + 6H2O(g) a) 469.7 g b) 300.6 g c) 250.0 g d) 3.406 g e) 2.180 g
Step by Step Solution
There are 3 Steps involved in it
Detailed Explanation Answer 1 Combustion is a chemical reaction that typically involves a hydrocarbon reacting with oxygen to produce carbon dioxide and water Balancing a chemical equation is fundamen... View full answer

Get step-by-step solutions from verified subject matter experts


