Question: Given the following combustion data for three different fuels: CH4 (g), CH (g) and CH3OH (1), CH4 (g) + 2 O2 (g) CO2 (g) +
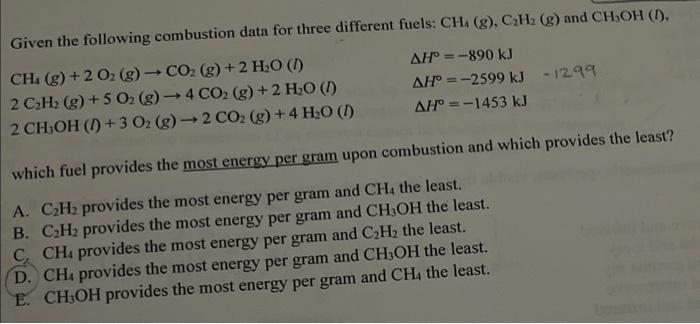
Given the following combustion data for three different fuels: CH4(g),C2H2(g) and CH3OH(D), CH4(g)+2O2(g)CO2(g)+2H2O(l)2C2H2(g)+5O2(g)4CO2(g)+2H2O(I)H=2599kJ12992CH3OH(l)+3O2(g)2CO2(g)+4H2O(l)H=1453kJ which fuel provides the most energy per gram upon combustion and which provides the least? A. C2H2 provides the most energy per gram and CH4 the least. B. C2H2 provides the most energy per gram and CH3OH the least. C. CH4 provides the most energy per gram and C2H2 the least. D. CH4 provides the most energy per gram and CH3OH the least. E. CH3OH provides the most energy per gram and CH4 the least
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


