Question: For a certain reaction, it was found that 10 AH = -70 kJ/mol and AS= 54.5 J/K.mol, find the value of the standard free energy
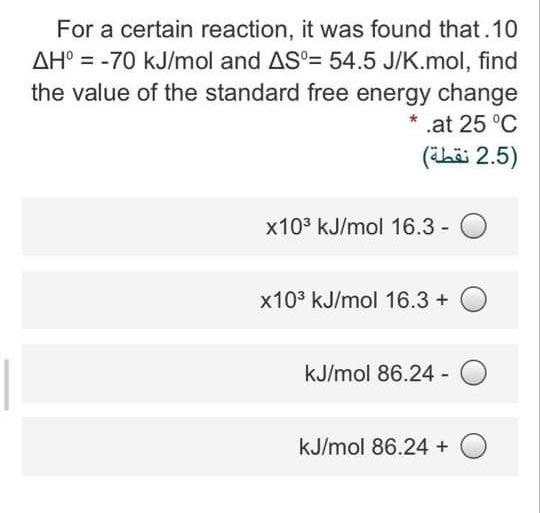

For a certain reaction, it was found that 10 AH = -70 kJ/mol and AS= 54.5 J/K.mol, find the value of the standard free energy change .at 25C (ibr 2.5) x103 kJ/mol 16.3 - O x103 kJ/mol 16.3 + 0 kJ/mol 86.24 - 0 kJ/mol 86.24 + O For the following reaction A+ 3B, 2AB.8 AH'=-50 kJ/mol what is the value of AS of the universe at 25 * C, IF AS of the reaction = -195 J/K.mol (ibai 2.5) J/K.mol 194.8 -O J/K.mol 27.3 + O J/K.mol 27.2 - 0 J/K.mol 167.78 + O
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


