Question: For a certain reaction, it .10 was found that AH = 120.5 kJ/mol and AS= 181.5 J/K.mol, find the value of the standard free energy
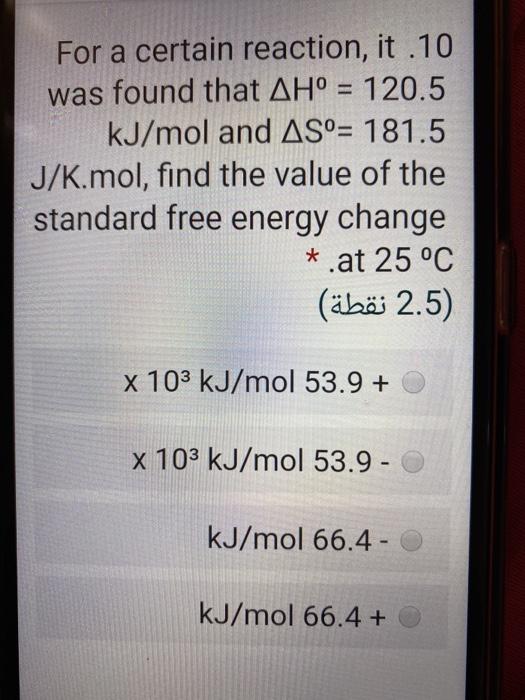
For a certain reaction, it .10 was found that AH = 120.5 kJ/mol and AS= 181.5 J/K.mol, find the value of the standard free energy change *.at 25 C (2.5 ) x 103 kJ/mol 53.9 + x 103 kJ/mol 53.9 - kJ/mol 66.4 - kJ/mol 66.4 +
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


