Question: For a closed system with no heat transfer, the change in entropy must be negative. (T/ F) Increasing the boiler pressure increases the average temperature
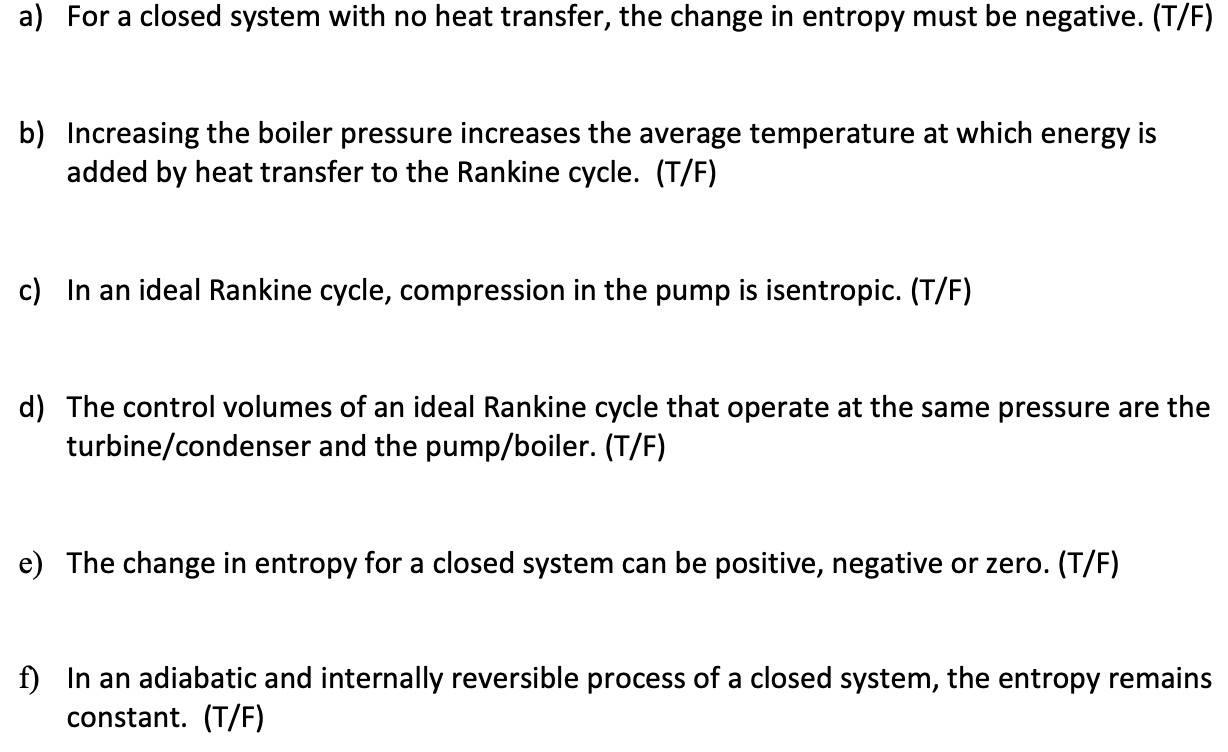
For a closed system with no heat transfer, the change in entropy must be negative. (T/ F) Increasing the boiler pressure increases the average temperature at which energy is added by heat transfer to the Rankine cycle. (T/F) In an ideal Rankine cycle, compression in the pump is isentropic. (T/F) The control volumes of an ideal Rankine cycle that operate at the same pressure are the turbinelcondenser and the pump/boiler. (T/F) The change in entropy for a closed system can be positive, negative or zero. (T/ F) In an adiabatic and internally reversible process of a closed system, the entropy remains constant. (T/ F)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


