Question: For a first order reaction A(g) 2B(g) + C(g) at constant volume and 300 K, the total pressure at the beginning (t = 0)
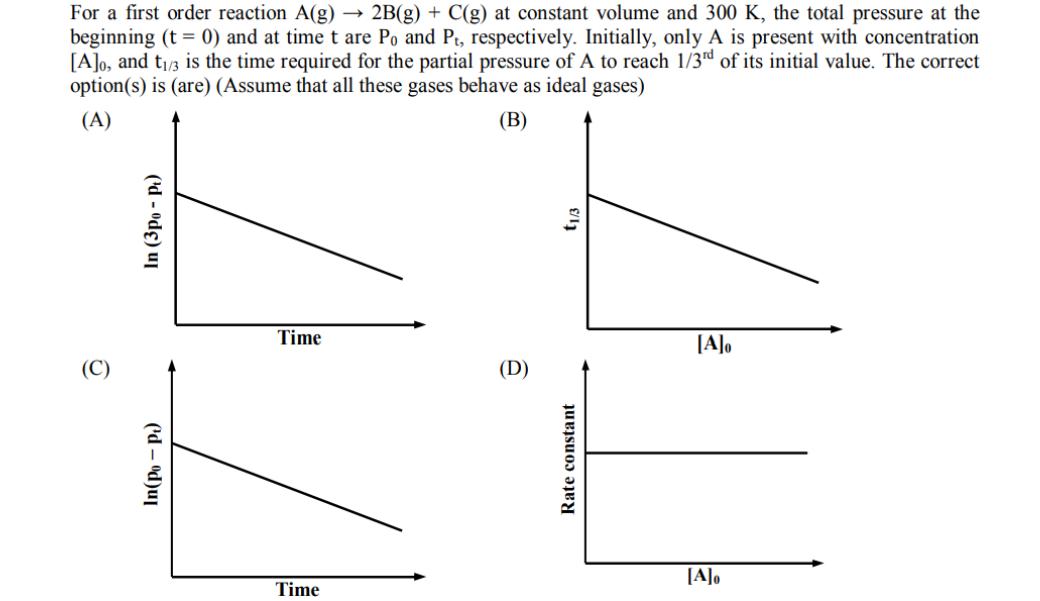
For a first order reaction A(g) 2B(g) + C(g) at constant volume and 300 K, the total pressure at the beginning (t = 0) and at time t are Po and Pt, respectively. Initially, only A is present with concentration [A]o, and t/3 is the time required for the partial pressure of A to reach 1/3rd of its initial value. The correct option(s) is (are) (Assume that all these gases behave as ideal gases) (B) (A) In (3po- pt) (d-od)ul Time Time (D) t1/3 Rate constant [A]o [A]o
Step by Step Solution
3.38 Rating (164 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


