Question: For a natural gas mixture below determine: a. Apparent molecular weight b. Specific gravity C. Gas density and specific volume at 1000 psia and 200F
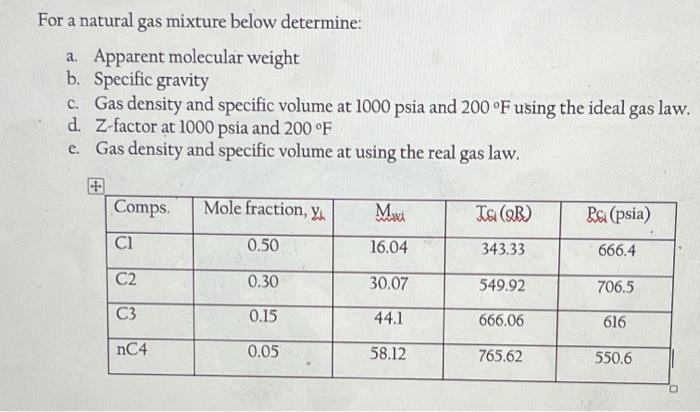
For a natural gas mixture below determine: a. Apparent molecular weight b. Specific gravity C. Gas density and specific volume at 1000 psia and 200F using the ideal gas law. d. Z-factor at 1000 psia and 200F e Gas density and specific volume at using the real gas law. Comps Mole fraction, you wi Ts (9) Rai (psia) ci 0.50 16.04 343.33 666.4 C2 0.30 30.07 549.92 706.5 C3 0.15 44.1 666.06 616 nC4 0.05 58.12 765.62 550.6
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


