Question: For a natural gas mixture below determine: a. Apparent molecular weight b. Specific gravity c. Gas density and specific volume at 1000 psia and 200F
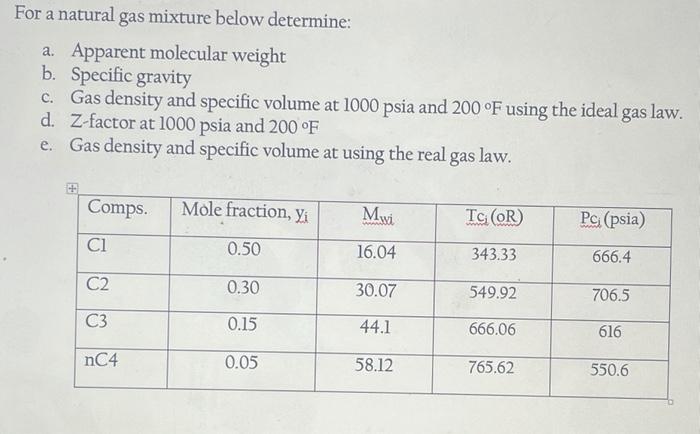
For a natural gas mixture below determine: a. Apparent molecular weight b. Specific gravity c. Gas density and specific volume at 1000 psia and 200F using the ideal gas law. d. Z-factor at 1000 psia and 200 F e. Gas density and specific volume at using the real gas law. Comps Mole fraction, Yi . TG (OR) Pc (psia) 0.50 16.04 343.33 666.4 C2 0.30 30.07 549.92 706.5 C3 0.15 44.1 666.06 616 nC4 0.05 58.12 765.62 550.6
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


