Question: For each reaction below, identify the atom oxidized, the atom reduced, the oxidizing agent, the reducing agent, the oxidation half reaction, the reduction half
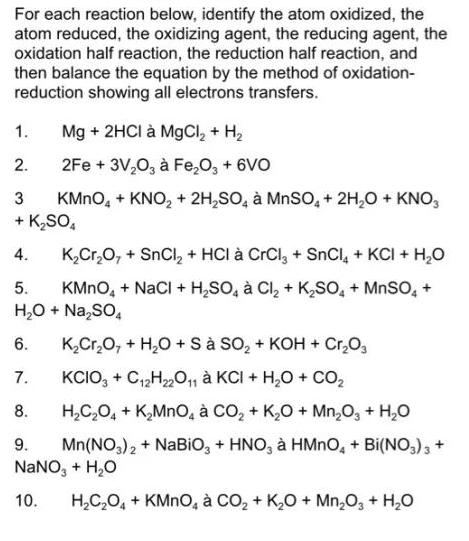
For each reaction below, identify the atom oxidized, the atom reduced, the oxidizing agent, the reducing agent, the oxidation half reaction, the reduction half reaction, and then balance the equation by the method of oxidation- reduction showing all electrons transfers. 1. Mg + 2HCI MgCl, + H, 2. 2Fe + 3V,0, Fe,O, + 6VO KMNO, + KNO, + 2H,SO, MnSO, + 2H,0 + KNO, + K,SO, 4. K,Cr,0, + SnCl, + HCI CrCl, + SnCl, + KCI + H,0 KMNO, + NaCI + H,SO, Cl, + Kso, + MnSO, + H,0 + Na,SO, 5. 6. K,Cr,O, + H,0 + S So, + KOH + Cr,0, KCIO, + CH0, KCI + H,O + CO2 H,C,0, + K,MnO, CO, + K,0 + Mn,0, + H,0 7. 8. 9. Mn(NO,)2 + NABIO, + HNO, HMNO, + Bi(NO,), + NANO, + H,O 10. H,C,0, + KMNO, CO, + K,0 + Mn,0, + H,0
Step by Step Solution
3.51 Rating (161 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


