Question: For the following cell Pb | PbCl(s) | PbCl(soln.) | AgCl(s) | Ag the potential at 298 K is 0.490 V and the variation
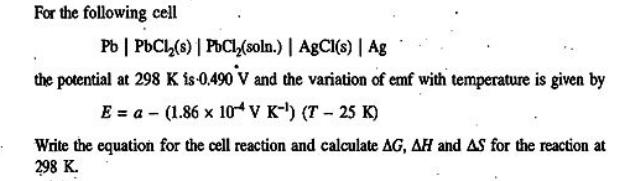
For the following cell Pb | PbCl(s) | PbCl(soln.) | AgCl(s) | Ag the potential at 298 K is 0.490 V and the variation of emf with temperature is given by E = a (1.86 x 104 V K-) (T-25 K) Write the equation for the cell reaction and calculate AG, AH and AS for the reaction at 298 K.
Step by Step Solution
3.49 Rating (159 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


