Question: For the cell Ag(s) | AgBr(s) | KBr(aq) | Hg,Br,(s) | Hg(l) the emfs at several temperatures are T/K 293 emf/V 0.066 3 298
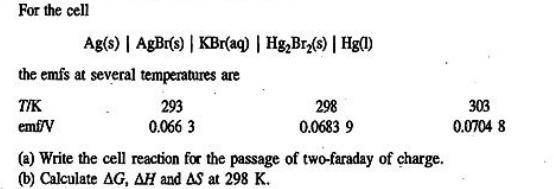
For the cell Ag(s) | AgBr(s) | KBr(aq) | Hg,Br,(s) | Hg(l) the emfs at several temperatures are T/K 293 emf/V 0.066 3 298 0.0683 9 (a) Write the cell reaction for the passage of two-faraday of charge. (b) Calculate AG, AH and AS at 298 K. 303 0.0704 8
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


