Question: For the reaction, X(s) = Y(s) + Z(g), the plot of In 2 versus - is given below (in 104 pe T solid line),
![]()
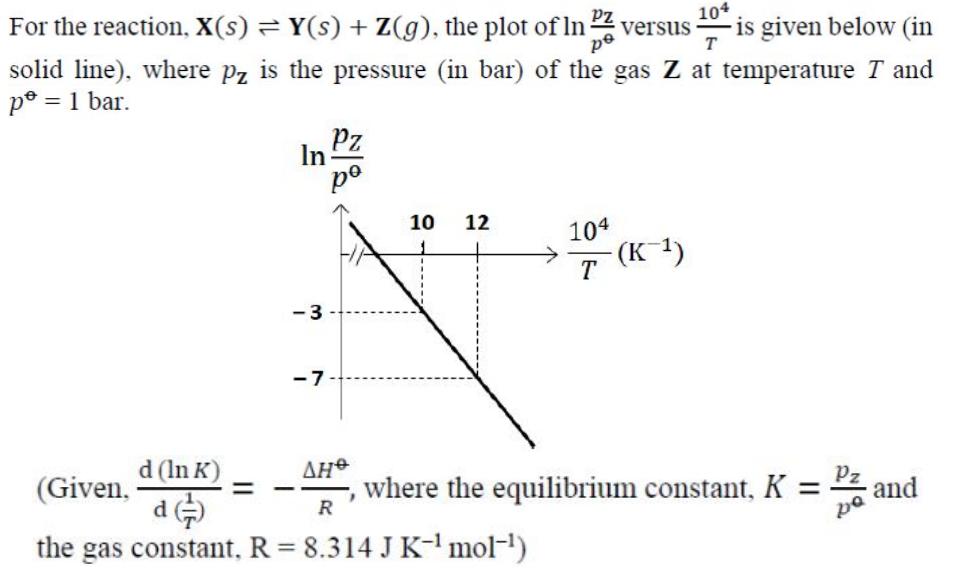
For the reaction, X(s) = Y(s) + Z(g), the plot of In 2 versus - is given below (in 104 pe T solid line), where pz is the pressure (in bar) of the gas Z at temperature T and po = 1 bar. d (ln K) d In = -3 Pz po -7 10 12 (Given, R the gas constant, R = 8.314 J K- mol-) 104 T -(K-) Pz where the equilibrium constant, K = pa and The value of standard enthalpy, AH* (in kJ mol-) for the given reaction is
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
The detailed answer for th... View full answer

Get step-by-step solutions from verified subject matter experts


