Question: Formic acid, H C O O H , is a weak acid with K a = 1 . 7 1 0 - 4 . What
Formic acid, is a weak acid with What is the molality of ions in a solution of formic acid in water? Do not neglect ionic activity coefficients.
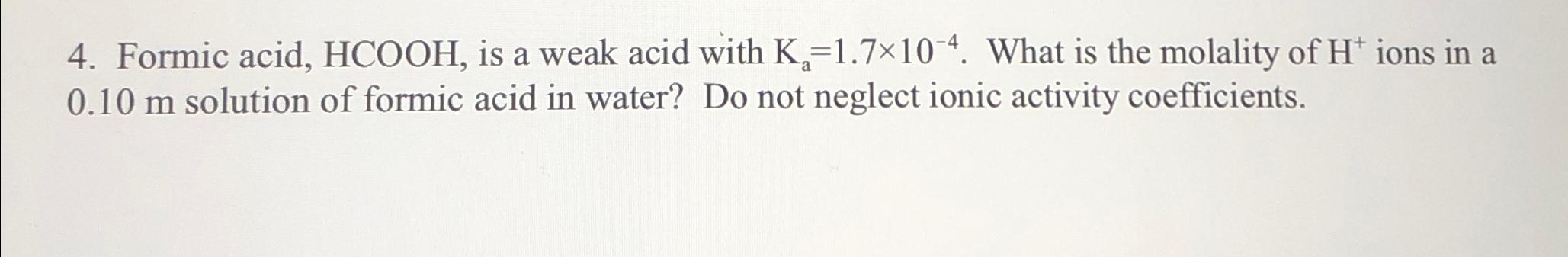
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


