Question: From the compounds listed, which will have the highest degree of ionization? (a) CH3CH2CH2COOH (b) CH3CH2CHBrCOOH (c) CH3CHBrCH2COOH (d) CH2BrCH2CH2COOH
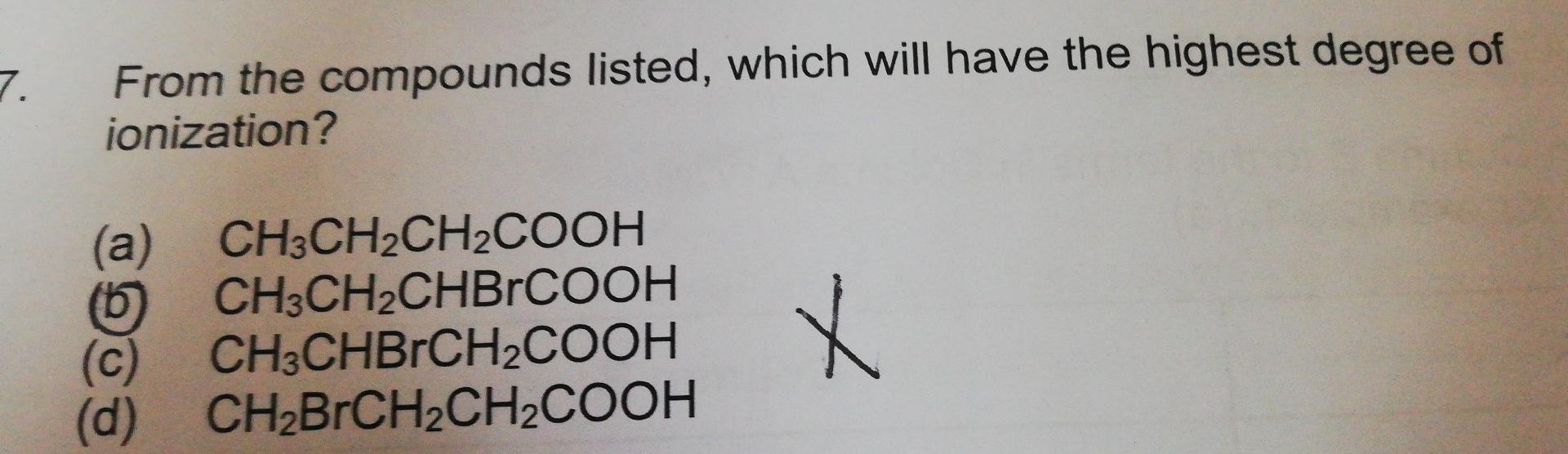
From the compounds listed, which will have the highest degree of ionization? (a) CH3CH2CH2COOH (b) CH3CH2CHBrCOOH (c) CH3CHBrCH2COOH (d) CH2BrCH2CH2COOH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


