Question: i need help with these questions. include arrow pushing illustrations 1) From among the following compounds, choose the two the yield the same carbocation on
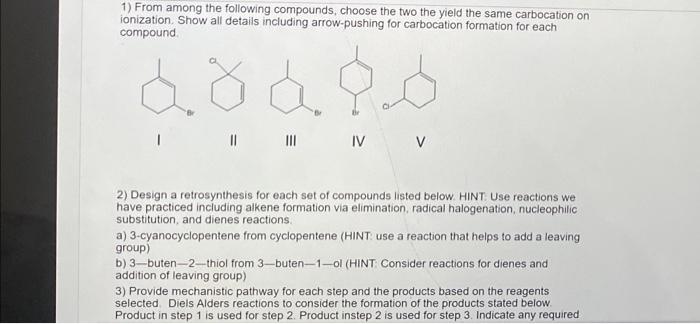
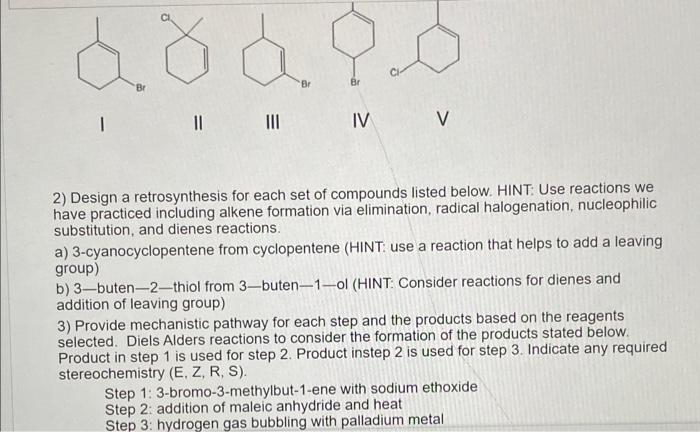
1) From among the following compounds, choose the two the yield the same carbocation on ionization. Show all details including arrow-pushing for carbocation formation for each compound 2) Design a retrosynthesis for each set of compounds listed below. HINT: Use reactions we have practiced including alkene formation via elimination, radical halogenation, nucleophilic substitution, and dienes reactions. a) 3-cyanocyclopentene from cyclopentene (HINT use a reaction that helps to add a leaving group) b) 3-buten-2 - thiol from 3-buten-1 -ol (HINT: Consider reactions for dienes and addition of leaving group) 3) Provide mechanistic pathway for each step and the products based on the reagents selected. Diels Alders reactions to consider the formation of the products stated below. Product in step 1 is used for step 2. Product instep 2 is used for step 3 . Indicate any required 2) Design a retrosynthesis for each set of compounds listed below. HINT: Use reactions we have practiced including alkene formation via elimination, radical halogenation, nucleophilic substitution, and dienes reactions. a) 3-cyanocyclopentene from cyclopentene (HINT: use a reaction that helps to add a leaving group) b) 3-buten-2-thiol from 3-buten - 1-ol (HINT: Consider reactions for dienes and addition of leaving group) 3) Provide mechanistic pathway for each step and the products based on the reagents selected. Diels Alders reactions to consider the formation of the products stated below. Product in step 1 is used for step 2. Product instep 2 is used for step 3 . Indicate any required stereochemistry (E, Z, R, S). Step 1: 3-bromo-3-methylbut-1-ene with sodium ethoxide Step 2: addition of maleic anhydride and heat 1) From among the following compounds, choose the two the yield the same carbocation on ionization. Show all details including arrow-pushing for carbocation formation for each compound 2) Design a retrosynthesis for each set of compounds listed below. HINT: Use reactions we have practiced including alkene formation via elimination, radical halogenation, nucleophilic substitution, and dienes reactions. a) 3-cyanocyclopentene from cyclopentene (HINT use a reaction that helps to add a leaving group) b) 3-buten-2 - thiol from 3-buten-1 -ol (HINT: Consider reactions for dienes and addition of leaving group) 3) Provide mechanistic pathway for each step and the products based on the reagents selected. Diels Alders reactions to consider the formation of the products stated below. Product in step 1 is used for step 2. Product instep 2 is used for step 3 . Indicate any required 2) Design a retrosynthesis for each set of compounds listed below. HINT: Use reactions we have practiced including alkene formation via elimination, radical halogenation, nucleophilic substitution, and dienes reactions. a) 3-cyanocyclopentene from cyclopentene (HINT: use a reaction that helps to add a leaving group) b) 3-buten-2-thiol from 3-buten - 1-ol (HINT: Consider reactions for dienes and addition of leaving group) 3) Provide mechanistic pathway for each step and the products based on the reagents selected. Diels Alders reactions to consider the formation of the products stated below. Product in step 1 is used for step 2. Product instep 2 is used for step 3 . Indicate any required stereochemistry (E, Z, R, S). Step 1: 3-bromo-3-methylbut-1-ene with sodium ethoxide Step 2: addition of maleic anhydride and heat
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


