Question: From the procodine 1. Fill your 400mL beaker about 3/4 full with DI water. Place several boiling stones in the water and place the beaker
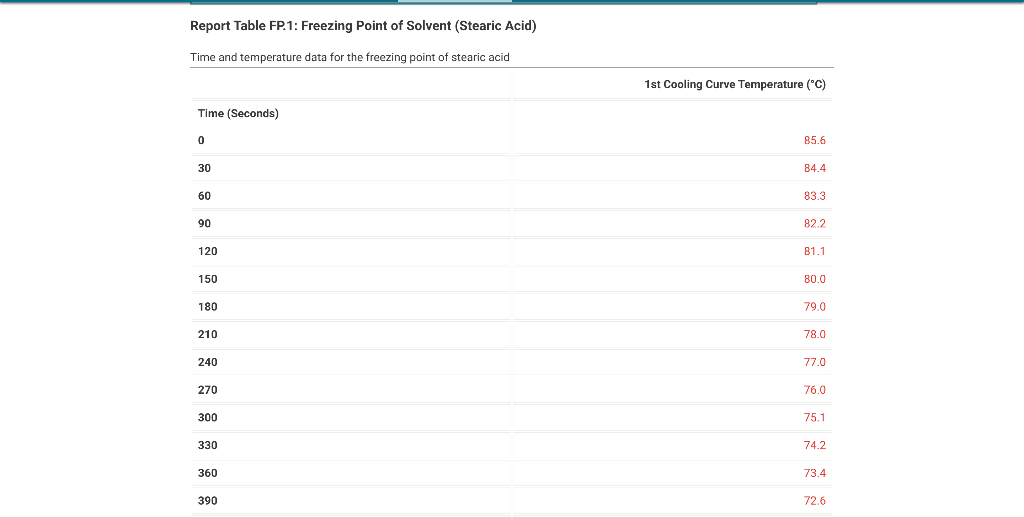

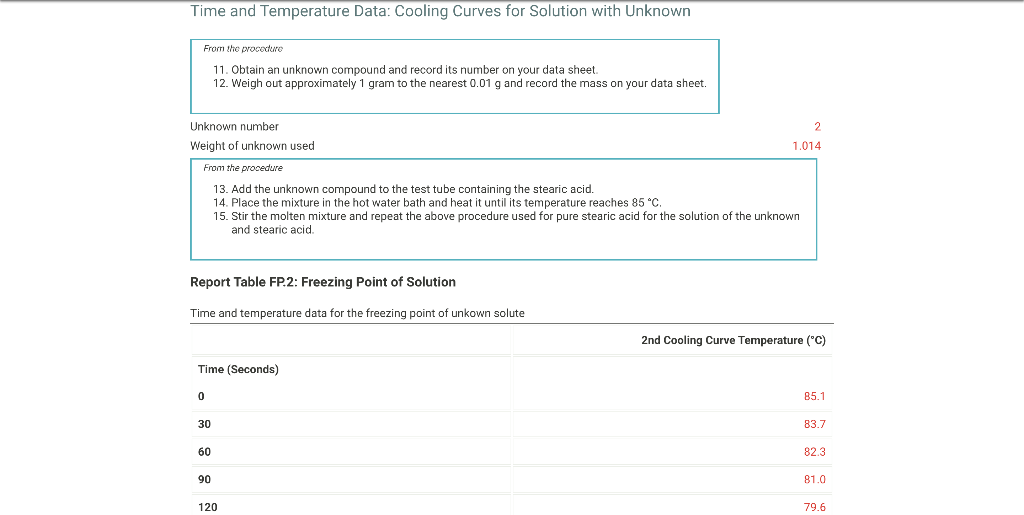

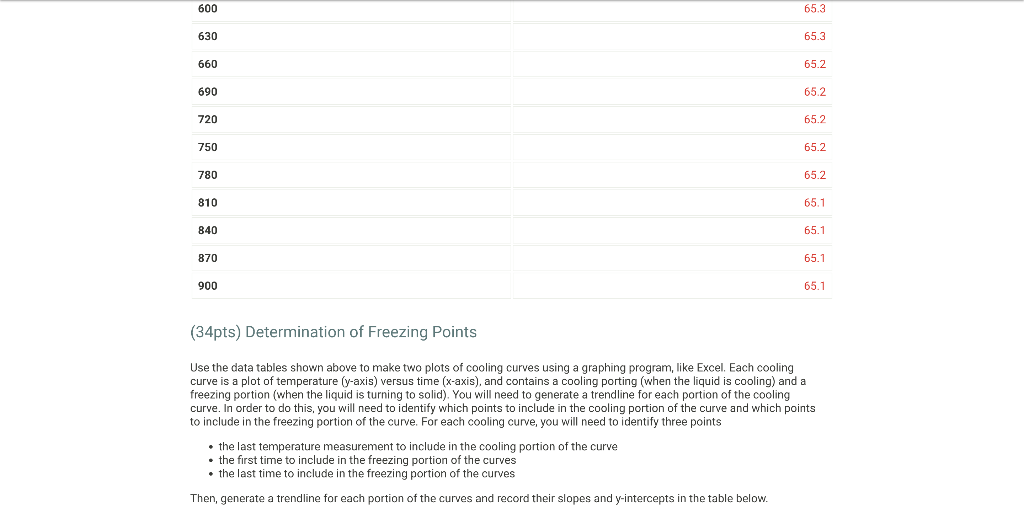
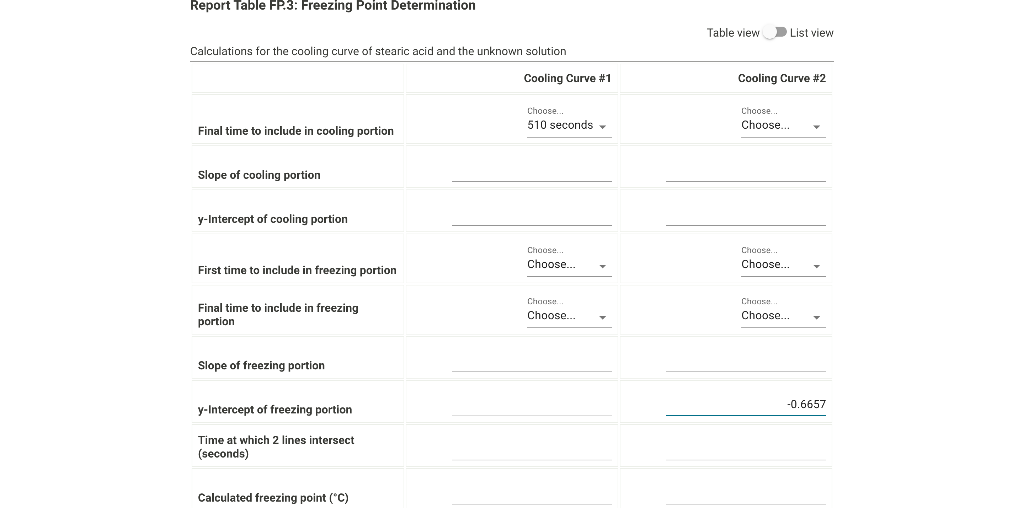

From the procodine 1. Fill your 400mL beaker about 3/4 full with DI water. Place several boiling stones in the water and place the beaker with the water on a hot plate. 2. Start heating the water to boiling using a mid-scale setting on the hot plate. 3. While the water bath is heating, stand up a test tube in your 30mL beaker and weigh both to the nearest 0.001g. Record the weight on your data sheet. 4. Repeat for a total of 5 trials. Weight of empty test tube and beaker (g) From the procedure 4. Add about 9 grams of stearic acid to the test tube and weigh the test tube, stearic acid and beaker to the nearest 0.001g. Record the weight on your data sheet. 5. Determine the mass of stearic acid using difference methods. Weight of test tube, beaker, and stearic acid (g) Report Table FP.1: Freezing Point of Solvent (Stearic Acid) \begin{tabular}{llr} 390 & 72.6 \\ \hline 420 & 71.8 \\ \hline 450 & 71.1 \\ \hline 480 & 70.5 \\ \hline 510 & 70.0 \\ \hline 570 & 69.6 \\ \hline 600 & 69.4 \\ \hline 630 & 69.3 \\ \hline 660 & 69.3 \\ \hline 690 & 69.3 \\ \hline 720 & 69.3 \\ \hline 750 & 69.3 \\ \hline 780 & 69.3 \\ \hline 810 & 69.2 \\ \hline 840 & 69.2 \\ \hline 870 & 69.2 \\ \hline \end{tabular} From the procedure 11. Obtain an unknown compound and record its number on your data sheet. 12. Weigh out approximately 1 gram to the nearest 0.01g and record the mass on your data sheet. Unknown number Weight of unknown used From the procedure 13. Add the unknown compound to the test tube containing the stearic acid. 14. Place the mixture in the hot water bath and heat it until its temperature reaches 85C. 15. Stir the molten mixture and repeat the above procedure used for pure stearic acid for the solution of the unknown and stearic acid. Report Table FP.2: Freezing Point of Solution Time and temperature data for the freezing point of unkown solute \begin{tabular}{llr} 150 & 78.3 \\ \hline 180 & 77.1 \\ \hline 210 & 75.8 \\ \hline 240 & 74.6 \\ \hline 270 & 73.5 \\ \hline 300 & 72.3 \\ \hline 330 & 71.2 \\ \hline 360 & 70.2 \\ \hline 390 & 69.2 \\ \hline 420 & 68.3 \\ \hline 450 & 67.5 \\ \hline 480 & 66.7 \\ \hline 510 & 66.1 \\ \hline 540 & 65.6 \\ \hline 570 & 65.4 \\ \hline 600 & 65.3 \\ \hline 630 & 65.3 \\ \hline \end{tabular} (34pts) Determination of Freezing Points Use the data tables shown above to make two plots of cooling curves using a graphing program, like Excel. Each cooling curve is a plot of temperature ( y-axis) versus time ( x-axis), and contains a cooling porting (when the liquid is cooling) and a freezing portion (when the liquid is turning to solid). You will need to generate a trendline for each portion of the cooling curve. In order to do this, you will need to identify which points to include in the cooling portion of the curve and which points to include in the freezing portion of the curve. For each cooling curve, you will need to identify three points - the last temperature measurement to include in the cooling portion of the curve - the first time to include in the freezing portion of the curves - the last time to include in the freezing portion of the curves Then, generate a trendline for each portion of the curves and record their slopes and y-intercepts in the table below. Calculations for the coolina curve of stearic acid and the unknown solution Report Table FP.4: Determination of molar mass
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


