Question: A) From the table you completed in Pre-lab question #1 from Exp. 25 in your lab manual, what is the initial SCN- concentration for solution
A) From the table you completed in Pre-lab question #1 from Exp. 25 in your lab manual, what is the initial SCN- concentration for solution 1?
B) From the table you completed in Pre-lab question #1 from Exp. 25 in your lab manual, what is the initial SCN- concentration for solution 4?
C) From the table you completed in Pre-lab question #1 from Exp. 25 in your lab manual, what is the equilibrium Fe(SCN)2+ concentration for solution 5?
D) Imagine that you plotted the absorbance and Fe(SCN)2+ concentration data from the table you’ve completed in Pre-lab Question #1 of Exp. 25 in your lab manual. By plotting this absorbance versus concentration data you would have a standard curve. Then, you take an absorption spectrum of a solution which has an unknown Fe(SCN)2+equilibrium concentration. The absorbance of the solution is determined to be 0.840. Which of the following equilibrium concentrations of Fe(SCN)2+ most closelycorresponds to the solution for which the absorbance was measured?
E)An equilibrium solution is prepared by mixing 2.750 mL of 0.001650 M SCN-, 5.000 mL of 0.001650 M Fe3+, and 2.750 mL of 0.05000 M HNO3. The equilibrium solution’s absorbance is determined to be 0.9150. Using this absorbance value and a standard curve, you determine that the equilibrium concentration of Fe(SCN)2+ is 0.0001830 M. Prepare an ICE table for the equilibrium mixture. Include the initial concentrations, changes in concentrations, and the equilibrium concentrations of Fe3+, SCN- and Fe(SCN)2+. Using the information in your ICE table, calculate the equilibrium constant. What is the value of the equilibrium constant?
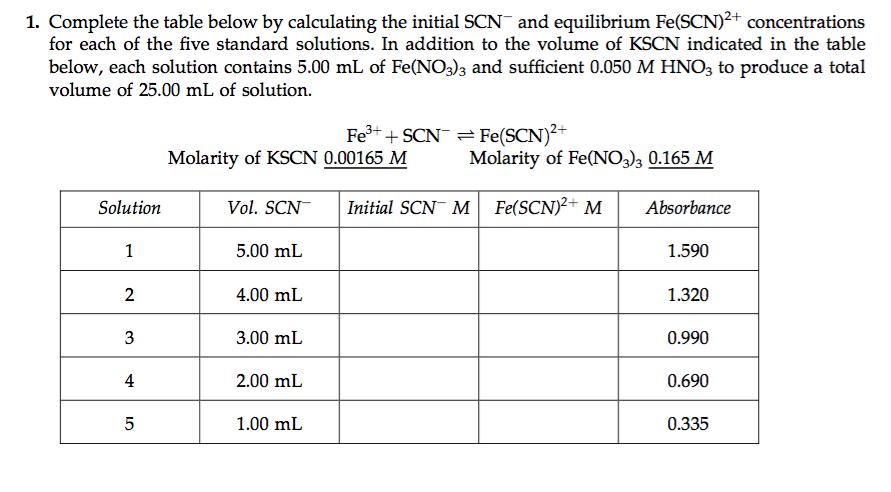
1. Complete the table below by calculating the initial SCN and equilibrium Fe(SCN)2+ concentrations for each of the five standard solutions. In addition to the volume of KSCN indicated in the table below, each solution contains 5.00 mL of Fe(NO3)3 and sufficient 0.050 M HNO3 to produce a total volume of 25.00 mL of solution. Solution 1 2 3 4 10 5 Molarity of KSCN 0.00165 M Vol. SCN 5.00 mL 4.00 mL 3.00 mL 2.00 mL Fe+ +SCN=Fe(SCN)+ 1.00 mL Molarity of Fe(NO3)3 0.165 M Initial SCN M Fe(SCN)+ M Absorbance 1.590 1.320 0.990 0.690 0.335
Step by Step Solution
3.57 Rating (168 Votes )
There are 3 Steps involved in it
A From the table you completed in Prelab question 1 from Exp 25 in your lab manual what is the initial SCN concentration for solution 1 Answer The ini... View full answer

Get step-by-step solutions from verified subject matter experts


