Question: Give solution as MATLAB script Problem 2. (30 points) The ideal gas law can represent the pressure-volume-temperature relationship of gases at low pressures (near atmospheric
Give solution as MATLAB script
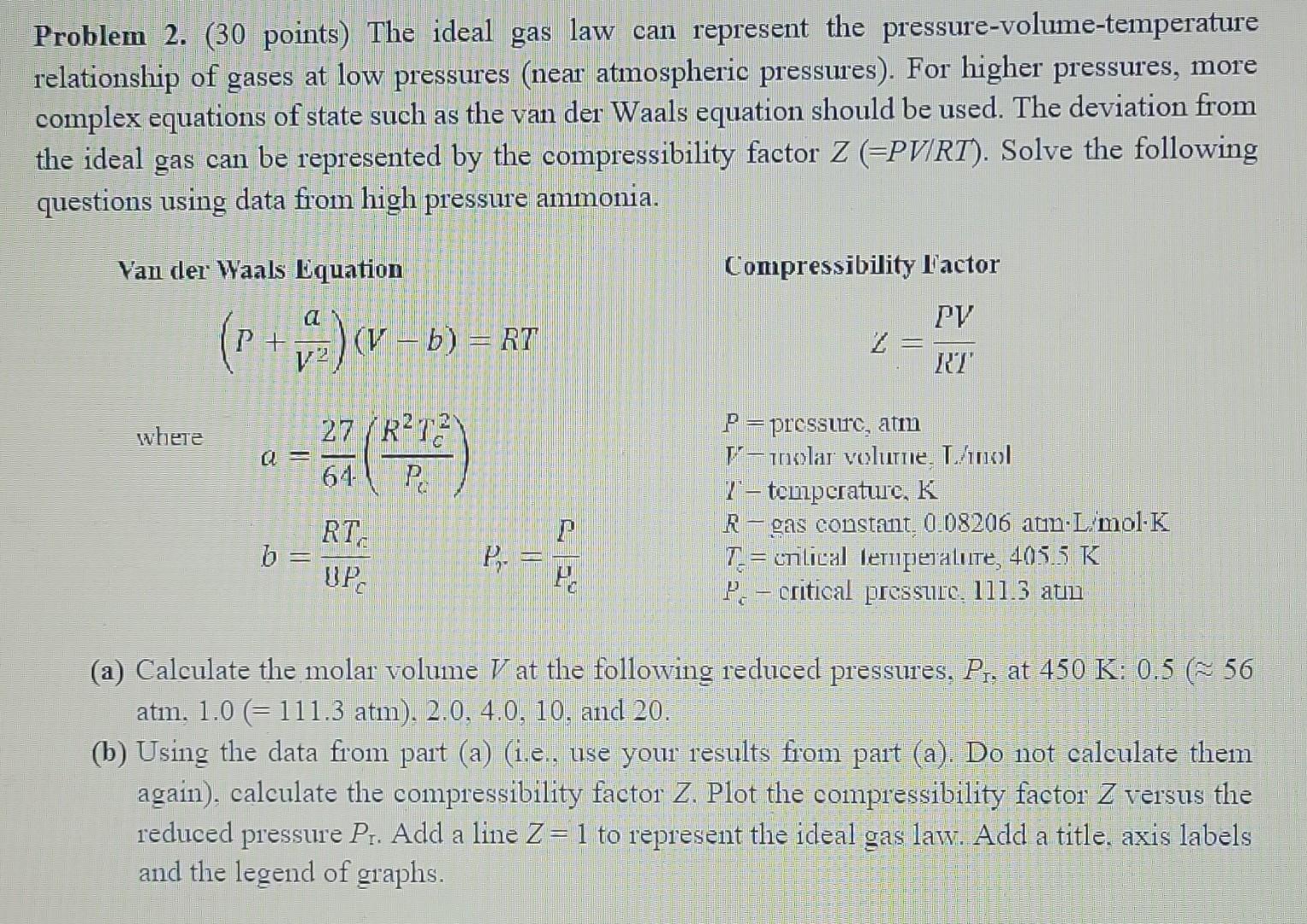
Problem 2. (30 points) The ideal gas law can represent the pressure-volume-temperature relationship of gases at low pressures (near atmospheric pressures). For higher pressures, more complex equations of state such as the van der Waals equation should be used. The deviation from the ideal gas can be represented by the compressibility factor Z(=PV/RT). Solve the following questions using data from high pressure ammonia. Yan der Waals Equation Compressibility Factor (P+V2a)(Vb)=RT =KIPV where a=6427(PcR2Tc2)P=pressure,atmV-nolarvolumie,L/olT-temperature,Kb=UPcRTcPr=HcPRgasconstant,0.08206atmLTc=crilicalleruperature,405.5KPccriticalpressule,111.3atm (a) Calculate the molar volume V at the following reduced pressures, PT, at 450K:0.5(56 atm, 1.0(=111.3atm),2.0,4.0,10, and 20 . (b) Using the data from part (a) (i.e., use your results from part (a). Do not calculate them again), calculate the compressibility factor Z. Plot the compressibility factor Z versus the reduced pressure Pr. Add a line Z=1 to represent the ideal gas law. Add a title, axis labels and the legend of graphs
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


