Question: give the matlab code fhat goes with this solution. please solve using matlab The ideal gas law for a gas at low temperature and pressure
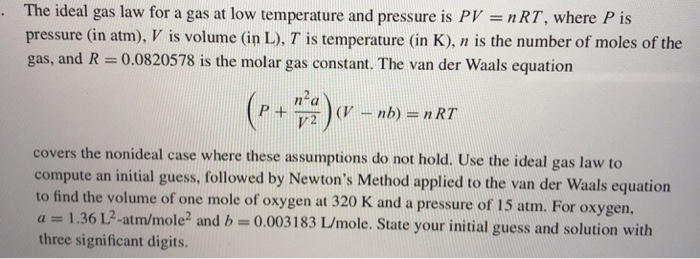
The ideal gas law for a gas at low temperature and pressure is PV nRT, where P is pressure (in atm), V is volume (in L), T is temperature (in K), n is the number of moles of the gas, and R = 0.0820578 is the molar gas constant. The van der Waals equation /2 covers the nonideal case where these assumptions do not hold. Use the ideal gas law to compute an initial guess, followed by Newton's Method applied to the van der Waals equation to find the volume of one mole of oxygen at 320 K and a pressure of 15 atm. For oxygen a 1.36 1?-atm/mole2 and b0.003183 L/mole. State your initial guess and solution with three significant digits
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


