Question: Given a buffer solution containing 10.0ml of 0.20MNaOAc and 10.0ml of 0.10MHOAc, what will be the change in pH of the buffer solution after the
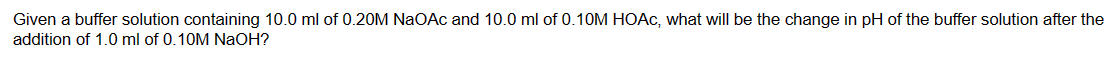
Given a buffer solution containing 10.0ml of 0.20MNaOAc and 10.0ml of 0.10MHOAc, what will be the change in pH of the buffer solution after the addition of 1.0ml of 0.10MNaOH
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


