Question: The acetic acid hydrolysi: was carried out in a series of four (4) equal tanks and also ir a series of four (4) different
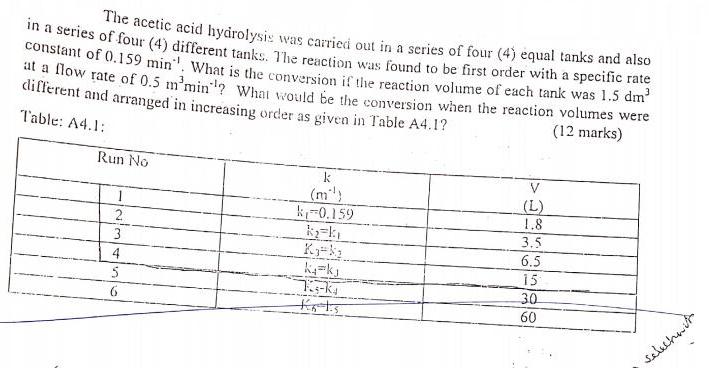
The acetic acid hydrolysi: was carried out in a series of four (4) equal tanks and also ir a series of four (4) different tanks. The reaction was found to be first order with a specific rate constant of 0.159 min". What is the conversion if the reaction volume of each tank was 1.5 dm t a How rate of 0.5 m'min"? What would be the conversion when the reaction volumes were different and arranged in increasing order as given in Table A4.1? (12 marks) Table: A4.1: Run No k V (m) k-0.159 (L) 1.8 2 3.5 3 6.5 4 15 30 6 60
Step by Step Solution
3.52 Rating (166 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


