Question: :Given the balanced equation: 2H2(g) + 02(g) -> 2H20(1)How many grams of H20 are formed if 9.00 mol H2(g) reacts completely with an excess of
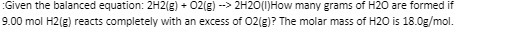
:Given the balanced equation: 2H2(g) + 02(g) -> 2H20(1)How many grams of H20 are formed if 9.00 mol H2(g) reacts completely with an excess of 02(g)? The molar mass of H20 is 18.0g/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


